Key Points
In women presenting with an initial diagnosis of TTP during pregnancy, cTTP was more common than acquired TTP.
Active monitoring and management during pregnancy results in positive pregnancy outcomes.
Abstract
Pregnancy can precipitate thrombotic thrombocytopenic purpura (TTP). We present a prospective study of TTP cases from the United Kingdom Thrombotic Thrombocytopenic Purpura (UK TTP) Registry with clinical and laboratory data from the largest cohort of pregnancy-associated TTP and describe management through pregnancy, averting fetal loss and maternal complications. Thirty-five women presented with a first TTP episode during pregnancy: 23/47 with their first congenital TTP (cTTP) episode and 12/47 with acute acquired TTP in pregnancy. TTP presented primarily in the third trimester/postpartum, but fetal loss was highest in the second trimester. Fetal loss occurred in 16/38 pregnancies before cTTP was diagnosed, but in none of the 15 subsequent managed pregnancies. Seventeen of 23 congenital cases had a missense mutation, C3178T, within exon 24 (R1060W). There were 8 novel mutations. In acquired TTP presentations, fetal loss occurred in 5/18 pregnancies and 2 terminations because of disease. We also present data on 12 women with a history of nonpregnancy-associated TTP: 18 subsequent pregnancies have been successfully managed, guided by ADAMTS13 levels. cTTP presents more frequently than acquired TTP during pregnancy and must be differentiated by ADAMTS13 analysis. Careful diagnosis, monitoring, and treatment in congenital and acquired TTP have assisted in excellent pregnancy outcomes.
Introduction
Thrombotic thrombocytopenic purpura (TTP) is an acute, rare, potentially life-threatening disorder, presenting with thrombocytopenia, hemolytic anemia, and clinical consequences of microvascular thrombosis, caused by deficiency of ADAMTS13.1,2 The majority of acute cases are acquired, autoantibody mediated, and characterized by low ADAMTS13 activity (<10%) and the presence of anti-ADAMTS13 IgG antibodies. A small proportion of TTP cases are attributable to congenital disease, with low ADAMTS13 activity (<10%) and no detectable antibody, confirmed by mutational analyses. Congenital TTP (cTTP) classically presents in neonates and children, but diagnosis in adulthood is described, such as pregnancy. In the South East England registry, 5% of more than 200 TTP episodes (congenital and acquired) occurred relating to pregnancy.3
The median age of acute TTP presentation is the third to fourth decade, typically affecting women. At least half of all acute episodes of TTP are in women of childbearing age. It is also recognized that pregnancy may be the initial, delayed presentation of congenital disease. There is limited data differentiating between TTP subtypes presenting in pregnancy and fetal and maternal outcomes in the index and subsequent pregnancies. Similarly, there is a paucity of data around the outcomes of pregnancy in women with a previous remote episode of acquired TTP.
Here we present the largest cohort of women with congenital and acquired TTP relating to pregnancy. In the cTTP patients, it represents their first TTP episode. The outcomes of TTP in pregnancy and the management of subsequent pregnancies in women with a history of acute TTP are also presented.
Methods
Cases were identified through referral to a single reference center (University College London) for laboratory diagnosis of TTP and recruited to the United Kingdom TTP (UK TTP) Registry from January 2009 to January 2013 (MREC 08/H0810/54). Eight cases were referred prior to the registry. Previous documentation relating to some of these cases has been published.4-6 Congenital cases presented with thrombocytopenia and microangiopathic hemolytic anemia and ADAMTS13 activity <10% with no evidence of an anti-ADAMTS13 autoantibody. ADAMTS13 activity was repeated, and upon confirmation of these results, mutational analysis was undertaken. Cases were divided into presentation of TTP before 20 weeks’ gestation, between 20 and 29 weeks’ gestation, and from 30 weeks’ gestation to 6 weeks postpartum. Spontaneous miscarriages, <12 weeks’ gestation, and termination of pregnancy, not clearly related to an acute TTP episode, were not included in the final numbers of the cohort presented. Furthermore, we present pregnancy outcome in women with previous TTP episodes unrelated to pregnancy. Approval was obtained from an institutional review board for this study (MREC 08/H0810/54). Informed consent was provided according to the Declaration of Helsinki.
Management of TTP presenting in pregnancy
Patients were managed according to the protocol in Figure 1.
Summary of the management of a patient presenting with acute TTP. All women require specialist obstetric review, regular fetal growth scan, and uterine artery Doppler monitoring. On achieving a platelet count of 50 × 109/L, start low-dose aspirin (LDA). Following an acute presentation, low-molecular-weight heparin (LMWH) thromboprophylaxis should be given. HBP, hypertension; LDH, lactate dehydrogenase; LFT, liver function test; MAHA, microangiopathic hemolytic anemia; PET, preeclampsia; PEX, plasma exchange; PI, plasma infusion; TMA, thrombotic microangiopathy.
Summary of the management of a patient presenting with acute TTP. All women require specialist obstetric review, regular fetal growth scan, and uterine artery Doppler monitoring. On achieving a platelet count of 50 × 109/L, start low-dose aspirin (LDA). Following an acute presentation, low-molecular-weight heparin (LMWH) thromboprophylaxis should be given. HBP, hypertension; LDH, lactate dehydrogenase; LFT, liver function test; MAHA, microangiopathic hemolytic anemia; PET, preeclampsia; PEX, plasma exchange; PI, plasma infusion; TMA, thrombotic microangiopathy.
Prior to 2009, subsequent pregnancies in women diagnosed with cTTP were managed with PEX every 2 weeks from positive pregnancy test. If there was a reduction in platelet count to <150 × 109/L, the frequency of PEX was increased to weekly until delivery. Women also received postpartum PEX, usually immediately after delivery, and were monitored for at least 6 weeks.
Since 2009, patients have been managed with PI from 8 to 10 weeks’ gestation, initially every 2 weeks, increasing to weekly from the second/early third trimester or if the platelet count drops below 150 × 109/L or increasing lactate dehydrogenase. All patients received Octaplas (Octapharma, UK) as plasma replacement.
ADAMTS13 assays
ADAMTS13 activity was determined by measuring the residual collagen binding activity of degraded exogenous von Willebrand factor7 until 2010, when analysis had been using the fluorescence resonance energy transfer assay8 (normal range: 60% to 123%). Anti-ADAMTS13 IgG antibodies were measured using an in-house enzyme-linked immunosorbent assay9 (normal range was <6.1% calculated as the 95th percentile of 49 normal healthy controls).
Genomic DNA was extracted from whole blood using an in-house ammonium chloride/dodecyl trimethyl ammonium bromide/ethanol precipitation method (Sigma-Aldrich, Dorset, UK). The 29 exons (and intronic boundaries) of ADAMTS13 were amplified using polymerase chain reaction with Biotaq DNA polymerase, 10× NH4 buffer, 50 mM MgCl2 solution, and 10 mM deoxyribonucleotide triPhosphate mix (Bioline, London, UK). The oligonucleotide primers (Invitrogen, Paisley, UK) and polymerase chain reaction conditions used are available on request. NG_011934.1 was used as the genomic DNA reference sequence; NM_139025.3 was used as the complementary DNA reference sequence. Amplification products were cleaned using Qiagen.
Role of the funding source
The UK TTP Registry was supported by a grant from the Medical Research Council from January 1, 2009, for 4 years. It provided funding for scientific and data support, capturing all cases of TTP in the UK. Included are a unique data set, admission, and remission samples, as well as a DNA biobank.
Results
Forty-seven women who had 91 pregnancies are included. Thirty-five women presented with de novo TTP in pregnancy. Twenty-three women had late-onset cTTP with no previous episodes of TTP before their presentation in pregnancy, and 12 women had acquired antibody-mediated TTP presenting for the first time in pregnancy. The remaining 12 women had suffered a previous episode of acute TTP unrelated to pregnancy and subsequently became pregnant.
cTTP presenting in pregnancy (n = 23)
There were 53 pregnancies, including previous pregnancies before TTP was considered, the index pregnancy when TTP was diagnosed, and management of pregnancies following diagnosis of cTTP (Table 1).
Late-onset cTTP presenting in pregnancy
| Case . | Pregnancy . | Treatment given . | Outcomes . |
|---|---|---|---|
| 1 | 1. 21/40* | PI/PEX | IUFD |
| 2. 37/40 | PEX 2 weekly from 12 wk and 6 wk postpartum | Live birth | |
| 2 | 1. 20/40 | Stillbirth | |
| 2. FTND | LDA | Live birth | |
| 3. 10/40 | Miscarriage | ||
| 4. FTND | LDA | Live | |
| 5. FTND* | LDA and LMWH | Live | |
| 3 | 1. 35/40* | PEX | Live birth but severe developmental delay |
| 2. 36/40 | BPL 8Y from 12/40, 20 U/kg 2 weekly, increasing to 30 U/kg weekly from 25/40; PEX pre- and postdelivery; LDA and LMWH | Live birth | |
| 3. 11/40 | Miscarriage | ||
| 4. 36/40 | PEX every 2 wk, increased to weekly from mid–second trimester | Live birth | |
| 4 | First trimester* | 10/7 PEX until TTP remission, continued 3×/wk PEX until delivery; LDA; LMWH | Live 34/40 |
| 5 | 17/40* | Daily PEX, LDA, LMWH, steroids, rituximab | Live 30+/40 |
| 6† | 1. 37/40 | Steroids | Stillbirth |
| 2. 32/40 | FFP and steroids | IUFD | |
| 3. 32/40 | Live birth | ||
| 4. 33/40 (twins) | IUFD 33/40 | ||
| 7 | 1. 20/40 | IUFD | |
| 2. 31/40* | Daily PEX until remission, then weekly until 37/40 and postpartum | Live birth 37/40 | |
| 3. 23/40 | Daily PEX until remission, then every 2 wk until 31/40, PEX increased to twice weekly; 5 PEX postpartum | Live birth 38/40 | |
| 8 | 1. 25/40* | IUFD | |
| 2. 35/40 | PEX every 2 wk from 12 wk | Live birth | |
| 3. 8/40 | Miscarriage | ||
| 4. 34/40 | PEX every 2 wk from 12 wk; changed to BPL 8Y from 20/40 to delivery | Live birth | |
| 9 | 40/40* | PEX, LDA | Live birth |
| 10 | 37/40* | PEX, LDA | Live birth |
| 11 | 1. 14/40 | IUFD | |
| 2. 19/40 | IUFD | ||
| 3. 20/40 | IUFD | ||
| 4. 6/40 | Miscarriage | ||
| 5. 36/40* | Daily PEX and steroids | Live birth 37/40 | |
| 6. 37/40 | LDA and LMWH throughout; PI from 10/40 every 2 wk until 20 wk, then continued weekly; 1 PEX pre- and postdelivery | Live birth | |
| 12 | 1. 38/40* | PEX, LDA | Live birth |
| 2. 37/40 | PEX every 2 wk from 12 wk, continued with PI every 2 wk from second trimester | Live birth | |
| 13 | 1. 39/40* | PEX | Live birth |
| 2. 38/40 | Weekly PI in third trimester, LMWH, and LDA | Live birth | |
| 14 | 1. 27/40 | Platelet transfusion | Live birth |
| 2. 34/40* | PEX, steroids, LDA, LMWH, IVIg | Live birth | |
| 3. 36/40 | BPL 8Y ×2/wk throughout pregnancy and 4 wk postpartum; 2× PEX predelivery | Live birth | |
| 15 | 32/40* | Daily PEX to TTP remission then weekly until delivery | Live birth 37/40 |
| 16 | 1. 20/40 (twins) | IVIg, prednisolone, platelets, PEX | IUFD 25/40 |
| 2. 39/40 | PI throughout pregnancies; platelets at delivery | Live birth | |
| 3. 37/40 | Live birth | ||
| 4. 37/40* | Live birth | ||
| 17 | 14/40* | Daily PEX until remission, then weekly PEX to delivery | Live birth 35/40 |
| 18 | (Known from positive FH) | LDA, PI every 2 wk, then weekly from 25/40 | 37/40 |
| 19 | 1. 20/40 | IUFD | |
| 2. 24/40 (twins)* | IUFD | ||
| 3. 6/40 | Miscarriage | ||
| 4. 37/40 | Plasma treatment ×2/wk from 12 wk | Live birth | |
| 20 | 33/40 | Steroids | Live birth |
| 21 | 1. 34/40 (twins) | Platelets | Live birth |
| 2. 28/40* | PEX | Live 29/40 | |
| 22 | 1. 24/40 | IUFD | |
| 2. 26/40 | IUFD | ||
| 3. 27/40 | IUFD | ||
| 4, 5, 6. 6-12/40 | 3 miscarriages | ||
| 7. 27/40 | Live birth | ||
| 8. 20/40* | LDA, LMWH, PI, PEX | IUFD 28/40 | |
| 23† | 36/40 | Live birth |
| Case . | Pregnancy . | Treatment given . | Outcomes . |
|---|---|---|---|
| 1 | 1. 21/40* | PI/PEX | IUFD |
| 2. 37/40 | PEX 2 weekly from 12 wk and 6 wk postpartum | Live birth | |
| 2 | 1. 20/40 | Stillbirth | |
| 2. FTND | LDA | Live birth | |
| 3. 10/40 | Miscarriage | ||
| 4. FTND | LDA | Live | |
| 5. FTND* | LDA and LMWH | Live | |
| 3 | 1. 35/40* | PEX | Live birth but severe developmental delay |
| 2. 36/40 | BPL 8Y from 12/40, 20 U/kg 2 weekly, increasing to 30 U/kg weekly from 25/40; PEX pre- and postdelivery; LDA and LMWH | Live birth | |
| 3. 11/40 | Miscarriage | ||
| 4. 36/40 | PEX every 2 wk, increased to weekly from mid–second trimester | Live birth | |
| 4 | First trimester* | 10/7 PEX until TTP remission, continued 3×/wk PEX until delivery; LDA; LMWH | Live 34/40 |
| 5 | 17/40* | Daily PEX, LDA, LMWH, steroids, rituximab | Live 30+/40 |
| 6† | 1. 37/40 | Steroids | Stillbirth |
| 2. 32/40 | FFP and steroids | IUFD | |
| 3. 32/40 | Live birth | ||
| 4. 33/40 (twins) | IUFD 33/40 | ||
| 7 | 1. 20/40 | IUFD | |
| 2. 31/40* | Daily PEX until remission, then weekly until 37/40 and postpartum | Live birth 37/40 | |
| 3. 23/40 | Daily PEX until remission, then every 2 wk until 31/40, PEX increased to twice weekly; 5 PEX postpartum | Live birth 38/40 | |
| 8 | 1. 25/40* | IUFD | |
| 2. 35/40 | PEX every 2 wk from 12 wk | Live birth | |
| 3. 8/40 | Miscarriage | ||
| 4. 34/40 | PEX every 2 wk from 12 wk; changed to BPL 8Y from 20/40 to delivery | Live birth | |
| 9 | 40/40* | PEX, LDA | Live birth |
| 10 | 37/40* | PEX, LDA | Live birth |
| 11 | 1. 14/40 | IUFD | |
| 2. 19/40 | IUFD | ||
| 3. 20/40 | IUFD | ||
| 4. 6/40 | Miscarriage | ||
| 5. 36/40* | Daily PEX and steroids | Live birth 37/40 | |
| 6. 37/40 | LDA and LMWH throughout; PI from 10/40 every 2 wk until 20 wk, then continued weekly; 1 PEX pre- and postdelivery | Live birth | |
| 12 | 1. 38/40* | PEX, LDA | Live birth |
| 2. 37/40 | PEX every 2 wk from 12 wk, continued with PI every 2 wk from second trimester | Live birth | |
| 13 | 1. 39/40* | PEX | Live birth |
| 2. 38/40 | Weekly PI in third trimester, LMWH, and LDA | Live birth | |
| 14 | 1. 27/40 | Platelet transfusion | Live birth |
| 2. 34/40* | PEX, steroids, LDA, LMWH, IVIg | Live birth | |
| 3. 36/40 | BPL 8Y ×2/wk throughout pregnancy and 4 wk postpartum; 2× PEX predelivery | Live birth | |
| 15 | 32/40* | Daily PEX to TTP remission then weekly until delivery | Live birth 37/40 |
| 16 | 1. 20/40 (twins) | IVIg, prednisolone, platelets, PEX | IUFD 25/40 |
| 2. 39/40 | PI throughout pregnancies; platelets at delivery | Live birth | |
| 3. 37/40 | Live birth | ||
| 4. 37/40* | Live birth | ||
| 17 | 14/40* | Daily PEX until remission, then weekly PEX to delivery | Live birth 35/40 |
| 18 | (Known from positive FH) | LDA, PI every 2 wk, then weekly from 25/40 | 37/40 |
| 19 | 1. 20/40 | IUFD | |
| 2. 24/40 (twins)* | IUFD | ||
| 3. 6/40 | Miscarriage | ||
| 4. 37/40 | Plasma treatment ×2/wk from 12 wk | Live birth | |
| 20 | 33/40 | Steroids | Live birth |
| 21 | 1. 34/40 (twins) | Platelets | Live birth |
| 2. 28/40* | PEX | Live 29/40 | |
| 22 | 1. 24/40 | IUFD | |
| 2. 26/40 | IUFD | ||
| 3. 27/40 | IUFD | ||
| 4, 5, 6. 6-12/40 | 3 miscarriages | ||
| 7. 27/40 | Live birth | ||
| 8. 20/40* | LDA, LMWH, PI, PEX | IUFD 28/40 | |
| 23† | 36/40 | Live birth |
FFP, fresh frozen plasma; FH, family history; FTND, full term normal delivery; IUFD, in utero fetal death; IVIg, intravenous immunoglobulin; LDA, low dose aspirin; LMWH, low molecular weight heparin.
Denotes the pregnancy when TTP was diagnosed.
Denotes retrospective diagnosis.
cTTP was diagnosed in 20 women following de novo presentation in pregnancy. Two women had pregnancies affected by TTP but were diagnosed with cTTP later in life. The final case (case 18) had a diagnosis of cTTP made as part of family screening of her siblings with poor obstetric outcomes and acute TTP in pregnancy. Therefore, she had an actively managed index pregnancy.
Pregnancies in women with cTTP preceding the diagnosis.
Eighteen pregnancies preceded the diagnosis of cTTP. None of these pregnancies presented at <20 weeks’ gestation. Between 20 and 29 weeks’ gestation, there were 10 pregnancies in 7 women resulting in 2 live births (both at 27 weeks’ gestation) and 8 IUFDs. In presentations after 30 weeks’ gestation, there were 8 pregnancies in 4 women of which 5 resulted in live births (including 1 set of twins) and 3 resulted in IUFD (including 1 set of twins).
Outcome of the index pregnancy in which cTTP presented.
Three women, presenting before 20 weeks, were treated with PEX at presentation and throughout the remainder of pregnancy. All delivered live births in the third trimester. In women presenting between 20 and 29 weeks (n = 6), there was 1 live birth following emergency section cesarean section, presenting with intrauterine growth retardation (IUGR) at 28 weeks. However, 5 pregnancies, including 2 sets of twins, resulted in IUFD.
In women presenting with cTTP after 30 weeks’ gestation (n = 11), there were 11 live births and no fetal losses. Some, but not all, women received plasma therapy, relating to the diagnosis of acute TTP (Table 1).
Pregnancies following the diagnosis of cTTP.
Ten women had 15 live births after a diagnosis of cTTP was made. They were actively monitored and treated throughout pregnancy and the postpartum period. There were no maternal or fetal deaths, and all deliveries were in the third trimester. Maternal symptoms during pregnancy were reduced compared with their index cases, and headache was the only documented symptom following treatment during pregnancy.
Two patients have been treated successfully with BPL 8Y (BioProducts Laboratory, Elstree, Hertfordshire, UK) during pregnancy, 1 because of severe anaphylaxis to plasma, and the other at the patient’s request. BPL is an intermediate purity factor VIII concentrate, which contains a relatively high concentration of ADAMTS13.10 Both pregnancies resulted in a successful outcome. In all cases, delivery was induced by 38 weeks’ gestation, in view of our observed increased risk of complications later in pregnancy in cTTP.
Five women with cTTP that presented initially in pregnancy have had subsequent episodes of TTP outside pregnancy, despite having no prior symptoms. All 5 of these women now receive maintenance PI. One patient (case 23) presented in her early 50s with recurrent stroke, and cTTP was confirmed during this admission but was retrospectively evident in her single pregnancy 28 years previously. Case 6 had strokes in her early 60s associated with thrombocytopenia and is on maintenance PI. She had no other documented thrombocytopenic episodes since her pregnancies.
Maternal symptoms associated with cTTP.
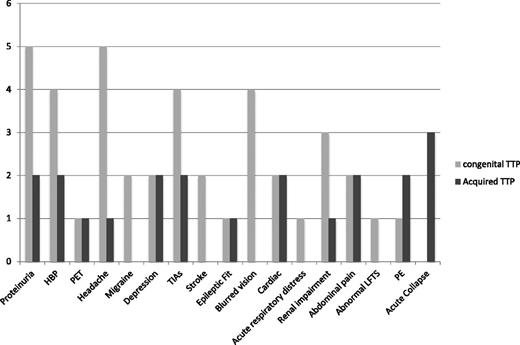
A range of clinical features was observed (Figure 2). Many of these were symptoms typically associated with TTP. However, there were features that could prompt a diagnosis of other pregnancy-associated TMAs, such as PET, proteinuria, and renal impairment. Neurologic symptoms including headache, migraine, blurred vision, and transient ischemic attacks and proteinuria were prominent in the cTTP women in pregnancy. However, symptoms alone cannot differentiate congenital from acquired TTP.
Symptoms documented in women presenting with pregnancy-associated TTP. Acute collapse, requiring intubation and ventilation; cardiac, chest pain and increased troponin T; PE, pulmonary emboli; TIA, transient ischemic attack.
Symptoms documented in women presenting with pregnancy-associated TTP. Acute collapse, requiring intubation and ventilation; cardiac, chest pain and increased troponin T; PE, pulmonary emboli; TIA, transient ischemic attack.
Mutational analysis of ADAMTS13 in pregnancy-associated onset of cTTP.
The ADAMTS13 mutations and single nucleotide ploymorphisms identified in these pregnancy-associated TTP cases are outlined (supplemental Table 1, available on the Blood Web site). The most striking feature is the predominance of the exon 24 missense mutation c.3178C>T, which was found in a homozygous (n = 6) or part of a compound heterozygous (n = 11) variation in 17 women. Two of the polymorphisms identified, R7W and A1033T, appeared coinherited with R1060W, present in 74% and 70% of cases, respectively. All the white women who presented with TTP episodes following pregnancy had heterozygous deletions in conjunction with another heterozygous mutation. We also identified 8 novel mutations, 4 point mutations (cases 13, 15, 16, and 17), and 4 deletions (cases 1, 2, 5, 14, and 16) (supplemental Table 1).
Acquired TTP presenting in pregnancy (n = 12)
Twelve women had acute pregnancy-associated TTP with an acquired phenotype, ADAMTS13 activity <0% at presentation, and detectable autoantibodies (IgG) to ADAMTS13 (median 44%, range 7.5% to 120%).
Index cases of acquired pregnancy-associated TTP.
Two women presented with TTP before 20 weeks’ gestation. One pregnancy resulted in IUFD. In the other, treatment resulted in complete remission of TTP, but the pregnancy was terminated because of unrelated fetal chromosomal abnormalities. Four women presented with TTP between 21 and 29 weeks’ gestation. These resulted in 1 live birth and 3 IUFDs. Six women presented after 30 weeks’ gestation; all of the pregnancies resulted in live births. Presenting clinical features were similar to cTTP patients (Figure 2). On diagnosis, women received PEX and immunosuppression with steroids as per standard TTP guidelines.11
Subsequent pregnancies in patients with a history of acquired TTP in pregnancy.
There were no maternal losses in any of the acquired cases, either at presentation or in subsequent pregnancies.
Subsequent to the index pregnancy, 6 women had a further 8 pregnancies resulting in 6 live births in the third trimester. ADAMTS13 activity at the onset of pregnancy was 46% (range 9% to 80%). One pregnancy was associated with an acute relapse of TTP at 6 weeks’ gestation resulting in termination of pregnancy. Another resulted in IUFD in the second trimester. Among the successful pregnancies, 1 required treatment during pregnancy and had a relapse postpartum. ADAMTS13 activity was normal at the beginning of pregnancy but reduced to <10% in the second trimester. Oral steroids and weekly PEX were started, and ADAMTS13 activity increased to 40% in the third trimester. The pregnancy continued uneventfully resulting in a live birth, but the patient relapsed 1 week following delivery. Two women received LDA only, and 1 received azathioprine and LMWH throughout pregnancy. ADAMTS13 activity was monitored throughout pregnancy in all cases.
Use of elective rituximab in nonpregnant state.
One woman was given preemptive rituximab when not pregnant. She had a history of 2 previous midtrimester stillbirths and an acute TTP relapse in both pregnancies. ADAMTS13 activity levels were <5% both during pregnancy and in the nonpregnant state, and she had high anti-ADAMTS13 IgG levels (120%). She only developed acute TTP during pregnancy. She received rituximab 375 mg/m2 ×6 doses electively, with reduction of the anti-ADAMTS13 IgG antibody to below the normal range and increased ADAMTS13 activity. She subsequently had 2 full-term normal deliveries, getting pregnant 12 months after rituximab therapy. During her first pregnancy, she had PEX every 2 weeks. In her second pregnancy, 1 year later, she had no plasma therapy, but monitoring of ADAMTS13 activity only. In both pregnancies, she received LDA and prophylactic LMWH.
Pregnancy in women with a previous history of TTP not associated with pregnancy (n = 12)
Twelve women with a history of acute acquired TTP, not related to pregnancy, have had 18 pregnancies including 2 sets of twins. In all cases, regular monitoring of ADAMTS13 levels was undertaken, at least each trimester, if the ADAMTS13 was normal. Any reduction in ADAMTS13 levels requires more frequent monitoring.All women received LDA ± LMWH prophylaxis. ADAMTS13 activity at the onset of pregnancy was 67% (range 43% to 89%). Women with normal ADAMTS13 activity at the beginning of pregnancy did not appear to have TTP-associated complications or relapse during pregnancy. However, in 1 case, there was a reduction in ADAMTS13 levels below 10%; intervention with elective PEX therapy was undertaken for a short period, but there was no clinical relapse. A further case had normal ADAMTS13 throughout her second pregnancy but developed acute lupus requiring immunosuppressives. She had no previous history of lupus. There were no TTP relapses in pregnancy. All delivered at term, and there was only 1 fetal loss, which was unrelated to TTP (attributable to β-hemolytic Streptococcus infection). The patient had received rituximab 3 months before her twin pregnancy, but it was not thought to have contributed to the fetal loss. Three other patients had received rituximab 6 to 48 months before conceiving with no complications for mother or fetus. One patient had TTP associated with HIV. She continued highly active antiretroviral therapy throughout pregnancy with normal ADAMTS13 activity and no complications.
Histopathology
Placental histology was available for review in 15 deliveries from 13 mothers. Of these, 3 were first trimester miscarriages. Of 5 untreated congenital cases (gestation 23-33 weeks), all had distal villous hypoplasia, and 3 of them had acute atherosis. A common feature was widespread placental ischemia with infarcts of varying ages. Of 4 treated congenital cases (34-37 weeks), all were normal, including a posttreatment delivery at 34 weeks in the same mother who had a delivery at 27 weeks with distal villous hypoplasia before treatment. Only 2 acquired cases had histology, an untreated IUFD at 23 weeks with hydrops and fetal anemia, but no other placental lesion, and 1 posttreatment normal placenta delivered at 34 weeks (Figure 3).
Placental histology in women with pregnancy-associated TTP and following treatment. (A) Pretreatment delivery at 28 weeks’ gestation: placenta showing an infarct (arrow). (B) Pretreatment delivery at 28 weeks’ gestation: distal villous hypoplasia, indicating ischemia (hematoxylin and eosin ×200). (C) Subsequent delivery following treatment at 36 weeks’ gestation: normal villi (hematoxylin and eosin ×200).
Placental histology in women with pregnancy-associated TTP and following treatment. (A) Pretreatment delivery at 28 weeks’ gestation: placenta showing an infarct (arrow). (B) Pretreatment delivery at 28 weeks’ gestation: distal villous hypoplasia, indicating ischemia (hematoxylin and eosin ×200). (C) Subsequent delivery following treatment at 36 weeks’ gestation: normal villi (hematoxylin and eosin ×200).
Results summary
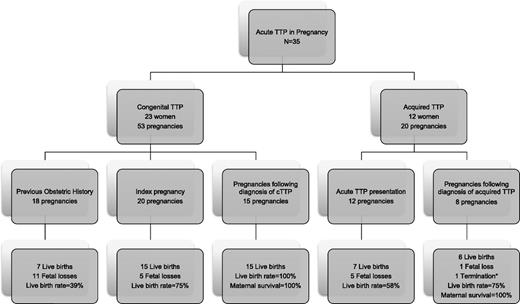
Overall, 23 of 35 (66%) of the women presenting with TTP for the first time in pregnancy had late-onset cTTP (Figure 4). One patient had already been diagnosed following a positive family history. In cTTP cases, preceding the diagnosis, fetal survival was 22/38 (58%) compared with 100% for actively managed cases following the diagnosis. Maternal survival was 100% in both circumstances within this cohort. Five out of 23 of the congenital cases (20%) required regular PIs following presentation with TTP in pregnancy, despite having had no previous history before pregnancy.
Summary of patients presenting with TTP in pregnancy. A summary of all the cases of women presenting with congenital and acquired TTP in pregnancy and the resulting fetal outcomes (* indicates termination <12 weeks because of severe refractory TTP).
Summary of patients presenting with TTP in pregnancy. A summary of all the cases of women presenting with congenital and acquired TTP in pregnancy and the resulting fetal outcomes (* indicates termination <12 weeks because of severe refractory TTP).
In acquired TTP, the overall fetal survival was 58% (7 of 12). Excluding those cases presenting in the postpartum period, fetal survival was 65% (5 of 9) in the index pregnancy and 75% (6 of 8) in subsequent pregnancies.
Considering all pregnancies together, both congenital and acquired cases, 46% of the 52 pregnancies associated with TTP presented after 30 weeks or postpartum, 38% between 20 and 29 weeks, and 15% <20 weeks’ gestation. The presentation of TTP was more common in the postpartum period in acquired compared with congenital cases, which presented more frequently in the third trimester.
Discussion
Pregnancy may be a precipitating factor in acute TTP, and there is a risk of relapse during subsequent pregnancies. To date, the phenotype of TTP during pregnancy has not been well documented, particularly relating to its heterogeneous clinical presentation and management in future pregnancies. TTP should be excluded in pregnancy-associated TMAs. We present the largest cohort of women with a history of TTP presenting in pregnancy and describe the outcomes of index pregnancies, as well as previous pregnancies and subsequent actively managed pregnancies.
In our cohort, 66% of women presenting with acute TTP in pregnancy or the immediate postpartum period had late onset, previously undiagnosed, congenital disease. In these cases, pregnancy was the initial and often the only precipitant of TTP. Throughout subsequent pregnancies, these women received elective ADAMTS13 replacement in conjunction with antithrombotic agents, resulting in uniformly successful outcomes with no further fetal losses. Indicators of the successful management include improved fetal growth and placental histology.
In contrast to previous evidence suggesting that the most common presentation of TTP was in the second trimester,12 our data document the majority of presentations after 30 weeks’ gestation. However, the authors of the previous study were using pooled published data from more than 50 years and acknowledged that few cases had ADAMTS13 assays to confirm the diagnosis. This further highlights the difficulty in distinguishing clinically between the obstetric TMAs, such as PET or aemolysis, elevated liver enzymes, low platelets.
Differentiation of congenital or acquired TTP using ADAMTS13 assays is required to guide the need for immunosuppressive therapy essential for acquired TTP but unnecessary in congenital disease. Furthermore, confirmation of a congenital phenotype is needed to ensure regular plasma therapy throughout subsequent pregnancies because of the high risk of fetal loss and relapsing TTP if untreated.
The outcome of pregnancy in women presenting with either congenital or acquired TTP is closely related to the gestation at presentation. Pregnancy loss typically occurred in the second trimester for both groups. Prompt diagnosis and treatment before 20 weeks was surprisingly associated with positive pregnancy outcomes. This suggests that early and later pregnancy presentations result in a situation where there is adequate placental function to avoid the development of severe IUGR, which is the most common cause of fetal loss. TTP is likely to be the only TMA presenting in the first trimester of pregnancy. However, maternal and fetal complications (such as hypertension and premature birth, respectively) remain a risk and mandate careful specialist multidisciplinary team monitoring and induction of delivery before full term.
From our data, TTP (especially previously undiagnosed congenital disease) should be considered in the differential diagnosis of, in particular, midtrimester pregnancy losses and atypical presentation of pregnancy-associated TMAs, and ADAMTS13 activity assay should be undertaken.
Previous published literature on cTTP and pregnancy is sparse.13,14 Recent data suggest the incidence of cTTP is 1 in 200 000 pregnancies.15 The French group describes a poor outcome in presentations in the second trimester, and in subsequent pregnancies, there was 100% relapse in cTTP women who received no plasma therapy. In a Japanese series of 9 women with cTTP, fetal loss was 50%, and all but 1 of the surviving babies was premature. The exception received PIs from 8 weeks’ gestation until term.16 Confirmation of the high rate of maternal and fetal complications has been presented. The symptoms may be difficult to differentiate from PET.17 The recommendation from our cohort for subsequent pregnancies in women with cTTP is regular PI (10 mL/kg) from 8 to 10 weeks’ gestation every 2 weeks in combination with LDA. PI usually increased to weekly from 20 weeks’ gestation and delivery aimed at 36 to 38 weeks’ gestation. If platelet counts drop below 150 × 109/L, an increase in therapy at any stage is required.
In our cohort, 17 of the 23 women with cTTP had a missense mutation, c3178C>T, in exon 24 (p.R1060W), which has previously been reported in adult-onset cTTP,4,5 but there is a higher proportion of cases with this abnormality than expected in this cohort. Similar findings were confirmed in the French cohort, with 8/10 cTTP cases having this heterozygous mutation.15 Functionally, the mutation causes severe intracellular retention of ADAMTS13 (<5% secretion), without affecting activity.4 The presence of R7W and A1033T polymorphisms was higher than reported in European normal controls (13% and 2%, respectively). Their action as positive modifiers of ADAMTS13 expression18 with this mutation may explain the quiescent clinical course until the stress of pregnancy.
However, the genetic defects alone do not completely explain the clinical presentation. None of these 23 cases had previous TTP episodes before pregnancy, but 20% required regular treatment following pregnancy for TTP. Four of the 5 women were white and had associated deletions rather than point mutations. Furthermore, even within the same molecular defect, clinical presentation differed (eg, cases 1 and 2). There were cases of fetal survival before cTTP was diagnosed and no treatment received. However, there were maternal symptoms described throughout pregnancy, which were associated with morbidity.
There were 8 further novel genetic defects identified that have not been previously described. Two of these mutations (in cases 13 and 16) may affect glycosylation sites, important in modulating ADAMTS13 activity and secretion.19
Successful pregnancy outcome can be achieved after acquired TTP is diagnosed in pregnancy.20-23 The risk of recurrence in subsequent pregnancies is reported to be ∼50%.12,24 In our cohort, there was only 1 postpartum relapse and no maternal deaths, which remains a risk.25 Regular monitoring of routine laboratory parameters and ADAMTS13 levels, which was not documented in other case series, allows for elective treatment.26
In women with a history of acquired nonpregnancy-related TTP, ADAMTS13 activity at the onset of subsequent pregnancy appears to be a good indicator of the risk of relapse.21 Our data suggest that women with previous acquired nonpregnancy-related TTP and normal ADAMTS13 activity at the onset of pregnancy, who maintain normal ADAMTS13 activity, do not usually relapse. Monitoring of ADAMTS13 activity in subsequent pregnancies in women with a history of acquired TTP is advisable with plasma therapy started electively if ADAMTS13 activity falls to <10%, to prevent microvascular thrombosis, which could affect placental function.27 There are no guidelines on frequency of ADAMTS13 activity assays, but for acquired TTP cases, we recommend monitoring at the start of pregnancy and at least in each trimester.
Where low ADAMTS13 preceded pregnancy, rituximab was used electively with successful pregnancy outcomes. Patients were advised to wait 12 months following rituximab before conceiving. However, some women became pregnant before this with no ill effects to mother or fetus. Indeed, waiting until normalization of CD19 lymphocyte levels, at ∼6 months with no detectable serum rituximab, may be satisfactory.28
A limiting factor of this work is the reporting of TTP cases to the registry, which is voluntary. The heterogeneous nature of presentations cannot be controlled for, but there was a standard therapeutic management of subsequent cases, particularly in cTTP.
In conclusion, pregnancy-associated TTP was attributable to late-onset cTTP in the majority of our cohort. These women require plasma therapy; PEX at acute presentation and infusion as prophylaxis throughout subsequent pregnancies. Early (<20 weeks) and late pregnancy (>30 weeks) presentations were associated with a good outcome, whereas presentations between 20 and 29 weeks’ gestation were more often associated with severe fetal IUGR and fetal death. A high proportion of the patients had the R1060W mutation, and 20% of the congenital cohort went on to require regular ADAMTS13 replacement following pregnancy. ADAMTS13 activity and inhibitor/antibody status is necessary for TTP subtype identification, management of subsequent pregnancies, and differentiation from other pregnancy-associated TMAs. Importantly, our data suggest that women with previous acute TTP may plan future pregnancies with appropriate specialist management.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
The authors thank Mrs Houda Webster for data management support; Fritz Scheiflinger, Baxter, for recombinant ADAMTS13 for the IgG assay; and Prof R. Gale for her expert support and advice in mutational analysis.
Authorship
Contribution: M.S. and A.R. designed the research; M.S., A.C., D.C., R.R., H.W., V.M., A.R., G.E., S.M., F.N.A., S.M., R.M., W.L., M.N., and P.O.B. provided patient samples/data; M.S., K.L., R.S.C., V.M., R.S., and M.U. performed laboratory analysis; M.S., A.R., H.W., M.T., R.S., and P.O.B. wrote the manuscript; and M.S., M.U., A.R., A.C., D.C., R.R., H.W., M.T., R.S., P.O.B., G.E., and F.N.A. reviewed the manuscript.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Marie Scully, 60 Whitfield St, London W1T 4EU, UK; e-mail: m.scully@ucl.ac.uk.



This feature is available to Subscribers Only
Sign In or Create an Account Close Modal