Key Points
Homing of T-lineage progenitors to the thymus is reduced after irradiation.
Chemokines limit thymic reconstitution after BMT.
Abstract
Development of T cells in the thymus requires continuous importation of T-lineage progenitors from the bone marrow via the circulation. Following bone marrow transplant, recovery of a normal peripheral T-cell pool depends on production of naïve T cells in the thymus; however, delivery of progenitors to the thymus limits T-lineage reconstitution. Here, we examine homing of intravenously delivered progenitors to the thymus following irradiation and bone marrow reconstitution. Surprisingly, following host conditioning by irradiation, we find that homing of lymphoid-primed multipotent progenitors and common lymphoid progenitors to the thymus decreases more than 10-fold relative to unirradiated mice. The reduction in thymic homing in irradiated mice is accompanied by a significant reduction in CCL25, an important chemokine ligand for thymic homing. We show that pretreatment of bone marrow progenitors with CCL25 and CCL21 corrects the defect in thymic homing after irradiation and promotes thymic reconstitution. These data suggest new therapeutic approaches to promote T-cell regeneration.
Introduction
T cells are an important component of the adaptive immune system in combating infection. Following bone marrow transplant (BMT), T cells are among the last of the hematopoetic lineages to recover, leaving patients susceptible to infection for a prolonged period.1,2 After BMT, peripheral T cells recover through 2 mechanisms: (1) thymus-independent homeostatic expansion of radioresistant cells and (2) thymus-dependent maturation of progenitor cells.3,4 Although both mechanisms increase T-cell numbers, the latter mechanism restores diversity of T-cell receptors and a functional peripheral T-cell population.5 However, the regeneration of T cells from the thymus is slow and can take years, which is further impeded by graft-versus-host disease and age-related thymic involution in humans.6-8 The reasons for the prolonged delay in thymus-derived T-cell reconstitution are unclear.
Under physiologic conditions, the thymus does not contain self-renewing progenitors, thus requiring importation of progenitors from the blood that originate in the bone marrow (BM).9 Although many BM stem and progenitor cells have T-lineage potential and differentiate into T cells when signaled through Notch, not all such progenitors migrate to the thymus.10,11 In mice, either chemokine receptors 7 (CCR7) or 9 (CCR9) support the trafficking of progenitors into the thymus.12,13 Progenitor homing via CCR9 has also been shown to be important in fish (medaka) and humans.14,15 The importance of CCR7 in physiologic thymic homing is less clear; however, in the absence of CCR9, cells can home using CCR7.13,16,17 Additionally, functional P-selectin glycoprotein ligand (PSGL-1) and integrins vascular cell adhesion molecule (VCAM)-1 and intercellular adhesion molecule (ICAM)-1 have been shown to be required for efficient thymic homing.18-20 Our understanding of molecules that mediate trafficking of progenitors to the normal thymus derives from unirradiated hosts; the effect of BMT conditioning on progenitor trafficking is not well understood.
BMT is preceded by conditioning regimens that most often include alkylating drugs and/or irradiation.21,22 In mice, when thymocytes are exposed to conditioning regimens, many of the hematopoietic cells in the thymus apoptose, and the debris is cleared by neutrophils and macrophages, resulting in reduced cellularity and decreased size.23 Although some T-lineage precursors can survive the irradiation and proliferate to become peripheral T cells in rodents, these cells are unable to maintain long-term T-cell output.24 After BMT, colonization of the BM by self-renewing hematopoietic stem cells (HSCs) eventually generates lymphoid progenitors that support thymic recovery; however, in mice, intrathymic niches remain unsaturated for a prolonged period after radiation and BMT, suggesting that the migration of progenitors to the thymus after BMT is a rate-limiting step in T-cell recovery.13 We examine whether irradiation reduces input of progenitors, which may contribute to delayed thymic-dependent T-lineage reconstitution after BMT.
In this study, we use a mouse model to examine homing of BM progenitors to the thymus. Among purified BM progenitors, only lymphoid-primed multipotent progenitors (LMPPs) and common lymphoid progenitors (CLPs) were verified as direct thymic homing precursors.25,26 We determine that very few—we estimate only 4 to 5 per 10 000 injected T-lineage competent progenitors—settle the normal thymus within 22 hours. After irradiation of the thymus, we find that the number of progenitors that settle reduces to below detectable levels. Radiosensitivity of thymic epithelial cells (TECs) reduces the total level of chemokine in the thymus, and chemokines are reduced on thymic endothelium. Ex vivo pretreatment of BM progenitors with chemokines prior to transplantation alters progenitors and increases homing from the circulation to the irradiated thymus. Together these data suggest that irradiation limits chemokine signals, slowing thymus-dependent T-lineage reconstitution after BMT. These data suggest a new strategy to boost T-lineage recovery after BMT in humans.
Materials and methods
Mice
C57BL/6 (CD45.2) and B6.Ly5SJL (CD45.1) female mice were purchased from the National Cancer Institute animal facility. CCR7-deficient and CCL25-deficient mice were purchased from The Jackson Laboratory. CCR9-deficient mice were a gift of Dr Paul Love (National Institutes of Health [NIH]). CCR7/CCR9-double deficient mice were generated by crossing these 2 strains. All live animal experiments were performed according to protocols approved by the Office of Regulatory Affairs of the University of Pennsylvania (Philadelphia, PA) in accordance with guidelines set by the NIH. Donor BM was taken from mice from 6 to 10 weeks old. All recipient mice were 4 to 7 weeks old.
Tissue preparation
BM cell suspensions were cleared of red blood cells using ammonium-chloride-potassium lysis buffer (Lonza). For cell sorting, BM was enriched for progenitors by incubation with anti-CD19 and anti-Gr-1 antibodies. Antibody-bound cells were removed with magnetic beads conjugated to goat anti-rat immunoglobulin (Ig)G (Qiagen). For dissection of thymi for the short-term homing assay, blood lymphocytes were removed from mice through exsanguination using a heparinized collection tube and intracardiac phophate-buffered saline (PBS) perfusion. Thymocytes were prepared as a single-cell suspension by manual dissection with forceps. For assessment of thymic settling progenitors, thymocytes were enriched for donor cells through depletion of single-positive and double-positive thymocytes with anti-CD4(GK1.5) and anti-CD8(53.6-7). For preparation of TECs and thymic endothelial cells, dissected thymocytes were incubated in 40 μg/mL Liberase thermolysin medium (Roche) and 200 μg/mL DNase I (Roche) in Hanks’ balanced salt solution for 30 minutes at 37°C with agitation. For enrichment of TECs and endothelial cells in preparation for cell sorting, samples were depleted of CD4+ and/or CD8+ cells as described above.
Flow cytometry and cell sorting
All antibodies were purchased from BD Biosciences, Biolegend, or eBiosciences. To exclude mature cells, a lineage cocktail of the antibodies was used including anti-B220(RA3-6B2), anti-CD19(1D3), anti-CD11b(M1/70), anti-Gr1(8C5), anti-CD11c(HL3), anti-NK1.1(PK136), anti-Ter119, anti-CD8α(53-6.7), anti-CD8β(53-5.8), anti-TCR-γδ(GL-3), anti-TCR-β(H57), and anti-CD3(2C11). Additional antibodies used were anti-CD45.1(A20), anti-CD45.2(104), anti-Thy1.2(53.2.1), anti-CD25(PC61.5), anti-CD4(GK1.5), anti-Kit(2B8), anti-IL-7Rα(A7R34), anti-Flt3(A2F10), anti-Sca1(D7), anti-VCAM-1(R1-2), anti-ICAM-1(3D2), anti-p-selectin(PB40.34), anti-CD31(390), and anti-EpCam(G8.8). Cells were sorted on a BD FACSAria (BD Biosciences) and sort purity was checked routinely. Dead cells were excluded with 4,6-diamidino-2-phenylindole. Doublets were excluded using forward scatter (FSC)-H by FSC-W and side scatter (SSC)-H by SSC-W parameters. Cell analysis was done on FACSCanto or LSRII (BD Biosciences), and data were analyzed using FlowJo version 9.3.1 (Tree Star). HSCs were defined as Lin−Kit+Sca1+Flt3−, multipotent progenitors (MPPs) as Lin−Kit+Sca1+Flt3lo, LMPPs as Lin−Kit+Sca1+Flt3hi, and CLPs as Lin−KitloSca1loFlt3+.27-30
Intravenous and intrathymic transfers
For intravenous transfers, between 1 × 106 and 4 × 107 T cell-depleted CD45.2 BM cells or sorted progenitors (or HSCs, MPPs, LMPPs, and CLPs sorted from 2 × 107 BM cells) were injected retro-orbitally into B6.Ly5SJL (CD45.1) recipients. Numbers of injected LMPPs and CLPs within 107 BM cells was calculated using flow cytometry prior to intravenous injection. For intrathymic adoptive transfer studies, 1 × 106 unfractionated BM cells or 2 × 103 to 1 × 104 sorted LMPPs (defined as BM Lin−Sca1+Kit+Flt3hi cells) were injected intrathymically as previously described. For irradiation studies, mice were inoculated (intravenously or intrathymically) with donor BM 2 to 4 hours after irradiation. Intrathymic injections were performed as previously described.13 Radiation was delivered at an average rate of 36.5 cGy/minute by a 137Cs source for a total dose of 600 or 900 cGy. Image-guided irradiation of the thymus was performed with a small animal radiation research platform with the assistance of Timothy W. Jenkins (Radiation Oncology, University of Pennsylvania).
In vitro culture of thymic progenitor cells
Recipient mice were exsanguinated and perfused with PBS, the thymus was removed, and a single-cell suspension was prepared in RPMI and 10% fetal bovine serum at 22, 44, or 72 hours after cell transfer. Samples were depleted of CD4- and CD8-expressing thymocytes and stained with antibodies to CD45.1 and CD45.2 for cell sorting. Donor cells were sorted and plated onto confluent wells of OP9-DL4 stroma (gift of Juan C. Zúñiga-Pflücker, University of Toronto), in the presence of 1 ng/mL of interleukin (IL)-7 and 5 ng/mL of fms-related tyrosine kinase 3 ligand (PeproTech). After 10 to 21 days of culture, donor-derived thymocytes were detected by flow cytometry using antibodies to CD25 and Thy1. For limit dilution analysis, all donor cells were sorted, and aliquots of sorted cells were added to 96-well OP9-DL4 cultures. Plating efficiency of progenitor cells was >70%.31 Wells containing a donor-derived T-lineage population (>20 Thy1+CD25+ cells) were considered positive. The frequency of progenitors in the sorted population was calculated using L-Calc software. Nonspecific cell loss due to depletion (typically 40-60%) was estimated by measuring the number of Lin−CD25+ DN3 cells before and after depletion, using flow cytometry to measure the frequency of DN3 cells combined with cell counts.
Real-time polymerase chain reaction
RNA was prepared from sorted populations using the RNeasy kit (QIAGEN). cDNA was generated using the Superscript II kit (Invitrogen). Real-time polymerase chain reaction (PCR) was performed using TaqMan Universal PCR Master Mix and primer/probe mixtures for Ccl21, Ccl25, Foxn1, and Gapdh (Applied Biosystems) and analyzed on a StepOnePlus Real Time PCR system (Applied Biosystems). Relative expression levels were normalized using Gapdh transcript levels and calculated using the 2–ΔΔCT method.
Tissue fixation and microscopy
Thymi were harvested in PBS and cryosectioned into 20-μm sections by the Cancer Histology Core (University of Pennsylvania Abramson Cancer Center). Tissues were fixed in acetone and stained with biotinylated anti-CCL25 (R&D), streptavidin-AF594 (Jackson), and anti-CD31-AF488 (Biolegend). Images were taken with a Zeiss LSM NLO/META with assistance from the Cell and Developmental Biology Microscopy Core (University of Pennsylvania). Quantification was performed with ImageJ on 6 images for each irradiated and unirradiated thymus. For quantitative analysis, arrows were drawn perpendicular through each CD31+ vessel without observing CCL25 fluorescence. Intensity plots were generated, and the 2 peaks of CD31 fluorescence (representing blood vessel walls) were selected. These plots were overlaid with the corresponding CCL25 fluorescence intensity plots and the intensities of CCL25 between the 2 peaks of CD31 fluorescence were summed. The average summed intensity of CCL25 fluorescence in CCL25KO thymi was subtracted.
Chemokine pretreatment
T-depleted BM (107) BM or 2 × 105 sorted Lin−Kit+Sca1+Flt3+ cells were suspended in α minimum essential medium and incubated with 500 ng/mL recombinant murine CCL21 (R&D) and 500 ng/mL recombinant murine CCL25 (R&D) for 30 minutes at 37°C. Media and chemokine cocktail were removed, and cells were resuspended in PBS or OP9 media for intravenous injection or in vitro culture, respectively. For α4 integrin blockade, anti-α4 (Clone R1-2; Biolegend) was incubated at 10 µg/mL at 37°C for 15 minutes prior to pretreatment.
Statistical analysis
For limiting dilution analysis assays, statistical calculations were performed using L-Calc software. P values were calculated using Microsoft Excel by the Student t test or GraphPad by Fisher’s exact test. All experiments were performed at least twice.
Results
LMPPs and CLPs each settle the normal thymus
Several hematopoietic progenitors have T-lineage potential; however, only intravenously injected LMPPs and CLPs rapidly generate intrathymic T cells, whereas earlier precursors such as multipotent HSCs and MPPs are delayed in differentiation.32 Hence, LMPPs and CLPs may each contain the immediate T-cell precursors that settle the thymus, although the low frequency and unknown cellular phenotype(s) of thymic settling progenitors makes the characterization of these T-lineage progenitors difficult.25,32,33 Once progenitors enter the thymus, the cells are exposed to Notch ligands and differentiate into early thymic progenitors (Lin−Kit+CD25−), the most primitive defined intrathymic T-cell precursor.34 To separate the ability of putative T-lineage progenitors to home to the thymus from later effects involving selective survival and proliferation, we measured homing to the thymus by circulating progenitors at early time points.35 BM (CD45.2) cells were intravenously injected into congenic mice (CD45.1). Thymocytes of recipient mice were prepared 22 to 72 hours later, and donor cells were isolated using a generous gate that included some host cells. Sorted cells were then plated on OP9-DL4 in the presence of cytokines IL-7 and Flt3 ligand. Between 2 and 3 weeks, cultures were analyzed for Thy1+CD25+ DN3 phenotype T-lineage precursors of donor origin (Figure 1A), confirming the presence of T-lineage competent progenitors. In these homing assays, we are measuring the presence or absence of the donor population; the percentage of donor cells inversely corresponds to the amount of inclusion of host cells from the sort.
LMPP and CLP populations contain thymic settling progenitors. (A) Schematic for experimental design of short-term homing assay. BM cells (CD45.2) were intravenously injected into congenically marked recipients (CD45.1). Twenty-two hours later, thymi were harvested from recipient mice, and donor cells were sorted and plated on OP9-DL4 cultures in the presence of IL-7 and Flt3 ligand. Cocultures were analyzed 2 to 3 weeks later. (B) Short-term homing assay of WT LMPPs or CCR7/CCR9 double knockout LMPPs intravenously injected into WT recipients (n = 2; P < .05 using Fisher’s exact test). (C) Short-term homing assay of HSCs, MPPs, LMPPs, and CLPs sorted from WT mice. Each population was intravenously injected into WT recipients. Cocultures were analyzed 2 to 3 weeks later (n = 3; P < .05 using Fisher’s exact test).
LMPP and CLP populations contain thymic settling progenitors. (A) Schematic for experimental design of short-term homing assay. BM cells (CD45.2) were intravenously injected into congenically marked recipients (CD45.1). Twenty-two hours later, thymi were harvested from recipient mice, and donor cells were sorted and plated on OP9-DL4 cultures in the presence of IL-7 and Flt3 ligand. Cocultures were analyzed 2 to 3 weeks later. (B) Short-term homing assay of WT LMPPs or CCR7/CCR9 double knockout LMPPs intravenously injected into WT recipients (n = 2; P < .05 using Fisher’s exact test). (C) Short-term homing assay of HSCs, MPPs, LMPPs, and CLPs sorted from WT mice. Each population was intravenously injected into WT recipients. Cocultures were analyzed 2 to 3 weeks later (n = 3; P < .05 using Fisher’s exact test).
To verify the validity of this assay to measure homing, LMPPs deficient in CCR7 and CCR9 were used as donor cells in a short-term homing assay. Compared with wild-type (WT) progenitors, CCR7/CCR9 double knockout progenitors are equally efficient at proliferating and differentiating in response to Notch signals in vivo and in vitro but are defective in homing to the thymus.13 T-lineage cells of donor CCR7/CCR9 double knockout origin were not detected in the cocultures (Figure 1B). Next, we examined the ability of different BM progenitors to home to the thymus. HSCs, MPPs, LMPPs, and CLPs were sorted from CD45.2 mice as lineage markers negative (Lin−) and identified using expression of Sca-1, Kit, and IL-7Rα and intravenously transplanted into congenic CD45.1 recipient mice.30,36 Only LMPPs and CLPs were each able to home to the thymus within 22 hours (Figure 1C), consistent with the known expression of CCR7, CCR9, and functional PSGL-1 on these progenitors, but not upstream HSCs and MPPs.12,13,19 To estimate the number of progenitors entering the thymus, we performed a short-term homing assay in which sorted cells were plated on OP9-DL4 at limiting dilution. We found that approximately 7 and 30 progenitors homed to the thymus from 1 × 107 and 4 × 107 BM cells, respectively (supplemental Figure 1 available on the Blood Web site). We calculated the fraction and number of LMPPs and CLPs within the injected total BM population and estimated that ∼4 to 5 of 10 000 LMPPs and CLPs can be shown to settle the thymus within 22 hours. Hence, very few intravenously injected cells directly home to the thymus, and this process requires expression of CCR9 and/or CCR7 by progenitors.
Irradiation profoundly reduces homing to the thymus
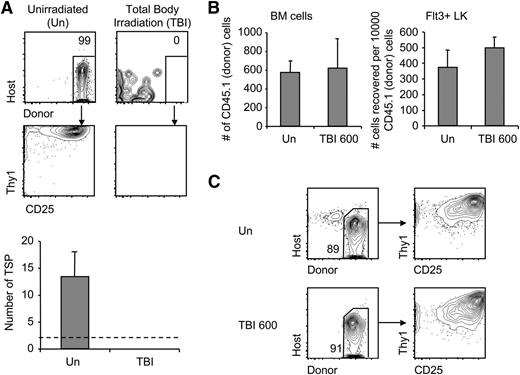
Previously, the thymus was thought to be hyper-receptive to progenitors due to increased donor engraftment in the thymus found in the weeks following irradiation; however, the increase in donor engraftment may have been due to increased proliferation within the thymus of rare thymic homing cells rather than a reflection of increased colonization.37 We therefore examined the impact of irradiation on homing. BM was injected into congenic recipient mice that were untreated or irradiated with 600 (sublethal) or 900 cGy (lethal). Surprisingly, when host thymi were examined 22, 44, or 72 hours later (for both 600 and 900 cGy), T-lineage precursors of donor origin were not detected in irradiated mice. Compared with controls, irradiated mice had at least a 10-fold decrease in progenitors that settle the thymus 72 hours after 900 cGy of irradiation (Figure 2A). The homing defect was equally evident when mice were given lower sublethal doses of 600 cGy (data not shown, also see Figure 6A). When homing was bypassed by intrathymic injection of BM or sorted Lin-Kit+Flt3+ progenitors, we found that progenitors survived the environment of the irradiated thymus (Figure 2B), and intrathymically injected donor BM cells containing T-progenitor cells sorted from either unirradiated and irradiated recipients differentiated into T-cell precursors on OP9-DL4 stromal cells with equivalent efficiency (Figure 2C). Together these data imply that thymic settling was harmed by irradiation rather than the survival or subsequent proliferation of settled cells.
Progenitor homing is profoundly reduced by irradiation conditioning. (A) Short-term homing assay was performed with unirradiated recipients or recipients receiving 600 or 900 cGy total body irradiation (TBI). Representative coculture plots are shown. P < .001 compared with unirradiated using Fisher’s exact test. The number of thymic settling progenitors was estimated using limit dilution analysis. Data are represented as mean ± standard error of the mean (SEM) (n = 3). Limit of detection (dotted line) is calculated from lowest number of donor cells that yielded positive results in short-term homing assay of unirradiated mice. (B) Mice were irradiated at 600 cGy or left untreated. Two hours later, 3 × 105 CD45.1 BM cells or sorted Flt3+Lin−Kit+ cells were intrathymically injected into recipient mice. Thymi were analyzed for donor cells by flow cytometry 22 hours later (n = 3). (C) Intrathymically injected donor BM cells (106) were sorted from irradiated or unirradiated mouse thymi and plated on OP9DL4. Cocultures were analyzed after 10 days (n = 2). Representative plots are shown.
Progenitor homing is profoundly reduced by irradiation conditioning. (A) Short-term homing assay was performed with unirradiated recipients or recipients receiving 600 or 900 cGy total body irradiation (TBI). Representative coculture plots are shown. P < .001 compared with unirradiated using Fisher’s exact test. The number of thymic settling progenitors was estimated using limit dilution analysis. Data are represented as mean ± standard error of the mean (SEM) (n = 3). Limit of detection (dotted line) is calculated from lowest number of donor cells that yielded positive results in short-term homing assay of unirradiated mice. (B) Mice were irradiated at 600 cGy or left untreated. Two hours later, 3 × 105 CD45.1 BM cells or sorted Flt3+Lin−Kit+ cells were intrathymically injected into recipient mice. Thymi were analyzed for donor cells by flow cytometry 22 hours later (n = 3). (C) Intrathymically injected donor BM cells (106) were sorted from irradiated or unirradiated mouse thymi and plated on OP9DL4. Cocultures were analyzed after 10 days (n = 2). Representative plots are shown.
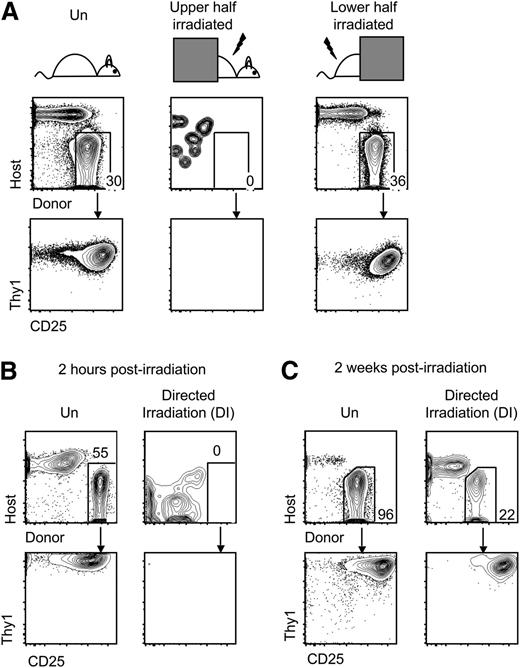
It was conceivable that nonthymic tissue damage caused by irradiation also may have remotely affected homing of progenitors to the thymus.38 Using a lead block to shield either the upper half or lower half of the recipients, we found that 900 cGy of irradiation of the upper half of the mouse blocked homing of progenitors, whereas irradiation of the lower half did not (Figure 3A), indicating that irradiation of nonthymic tissues was not sufficient to impair thymic settling. To ensure that the homing defect resulted from thymic irradiation, we used computed tomography scans to guide a collimator and direct a beam of irradiation 1.1 cm in diameter at the recipient thymus. Progenitor homing to the thymus was again blocked following targeted irradiation of the thymus (Figure 3B). The near-complete lack of thymic homing was not permanent, because some homing of progenitors to the irradiated thymus was evident after 2 weeks (Figure 3C). These results suggest that irradiation of thymus tissue directly impairs homing to the thymus.
Irradiation conditioning of the thymus reduces homing of progenitors. (A) Short-term homing assay was performed with unconditioned recipients or recipients receiving 900 cGy irradiation to the upper (includes thymus) or lower half (excludes thymus). Cocultures were analyzed by flow cytometry between 2 and 3 weeks later (n = 3; P < .05 comparing upper half to unconditioned using Fisher’s exact test). (B) Short-term homing assay was performed on unirradiated recipients or recipients receiving 900 cGy directed irradiation of the thymus (DI). Cocultures were analyzed between 2 and 3 weeks later (n = 3; P < .05 using Fisher’s exact test). (C) Short-term homing assay was performed on mice 2 weeks after mice were left unirradiated or irradiated with 900 cGy of directed thymic irradiation. Cocultures were analyzed between 2 and 3 weeks later (n = 2).
Irradiation conditioning of the thymus reduces homing of progenitors. (A) Short-term homing assay was performed with unconditioned recipients or recipients receiving 900 cGy irradiation to the upper (includes thymus) or lower half (excludes thymus). Cocultures were analyzed by flow cytometry between 2 and 3 weeks later (n = 3; P < .05 comparing upper half to unconditioned using Fisher’s exact test). (B) Short-term homing assay was performed on unirradiated recipients or recipients receiving 900 cGy directed irradiation of the thymus (DI). Cocultures were analyzed between 2 and 3 weeks later (n = 3; P < .05 using Fisher’s exact test). (C) Short-term homing assay was performed on mice 2 weeks after mice were left unirradiated or irradiated with 900 cGy of directed thymic irradiation. Cocultures were analyzed between 2 and 3 weeks later (n = 2).
Expression of determinants of homing on endothelial cells
Because both sublethal (600 cGy) and lethal (900 cGy) doses of irradiation ablated thymic homing, we chose to further examine the thymi of mice given sublethal doses, thus eliminating the need to prevent the death of the mice with BMT. The thymus is smaller after irradiation, with greatly reduced numbers of hematopoietic cells; however, we found that thymic endothelial cell numbers were unaffected after 600 cGy of irradiation (Figure 4A), consistent with reports of maintained endothelial numbers at other sites following irradiation.39,40 To determine the mechanism of the homing defect after BMT, we examined endothelial cells for molecules involved in progenitor cell homing. In addition to chemokine receptors, PSGL-1, lymphocyte function-associated antigen 1 (LFA-1) (αLβ2), and very late antigen-4 (VLA-4) (α4β1) on BM progenitors are each required for efficient homing to the thymus.18,20 We examined the respective binding partners on endothelial cells: P-selectin, ICAM-1, and VCAM-1 (Figure 4B-D). After 600 cGy irradiation, we found a modest increase in the number of P-selectin-positive endothelial cells, consistent with a previous report,41 as well as increased levels of ICAM-1 expression and maintenance of VCAM-1 expression. The results suggest that the homing defect is not likely due to defects intrinsic to endothelial cells.
Homing molecules on endothelial cells continue to be expressed after irradiation. Thymi were harvested from unirradiated mice and mice that had been irradiated with 600 cGy 24 hours prior. (A) Total thymic cellularity was enumerated. Enumeration of endothelial cells was determined by flow cytometric analysis of the CD45−CD31+ population (n = 3). (B) P-selectin, (C) ICAM-1, and (D) VCAM-1 expression was determined on CD31+ endothelial cells by flow cytometry. Shown are histograms gated on unirradiated CD45+ cells (gray), unirradiated CD31+ cells (solid black line), and irradiated CD31+ (dotted black line) (n = 3). Gating for P-selectin was established previously with P-selectin knockout mice. Data are represented as mean ± SEM. *P < .05 compared with unirradiated using the Student t test. (E) Enumeration of epithelial cells was determined by flow cytometric analysis of the CD45−EpCam+ TEC population (n = 3). (F) Short-term homing assay of WT cells into congenic irradiated WT or p53-deficient recipients (n = 2; P < .05 using Fisher’s exact test). Representative flow cytometric plots of cocultures are shown.
Homing molecules on endothelial cells continue to be expressed after irradiation. Thymi were harvested from unirradiated mice and mice that had been irradiated with 600 cGy 24 hours prior. (A) Total thymic cellularity was enumerated. Enumeration of endothelial cells was determined by flow cytometric analysis of the CD45−CD31+ population (n = 3). (B) P-selectin, (C) ICAM-1, and (D) VCAM-1 expression was determined on CD31+ endothelial cells by flow cytometry. Shown are histograms gated on unirradiated CD45+ cells (gray), unirradiated CD31+ cells (solid black line), and irradiated CD31+ (dotted black line) (n = 3). Gating for P-selectin was established previously with P-selectin knockout mice. Data are represented as mean ± SEM. *P < .05 compared with unirradiated using the Student t test. (E) Enumeration of epithelial cells was determined by flow cytometric analysis of the CD45−EpCam+ TEC population (n = 3). (F) Short-term homing assay of WT cells into congenic irradiated WT or p53-deficient recipients (n = 2; P < .05 using Fisher’s exact test). Representative flow cytometric plots of cocultures are shown.
Chemokines on thymic endothelial cells are reduced by irradiation
We then examined the thymus after sublethal doses of irradiation (600 cGy) in search of a mechanism for reduced homing after irradiation. We found that TECs, like hematopoietic cells, are radiosensitive (Figure 4E).42,43 To reduce irradiation-induced TEC apoptosis, we transplanted WT BM into irradiated p53-deficient recipients. We found that progenitors homed to irradiated thymus in which TEC numbers were maintained (Figure 4F), suggesting that TEC apoptosis may contribute to the defect in homing.
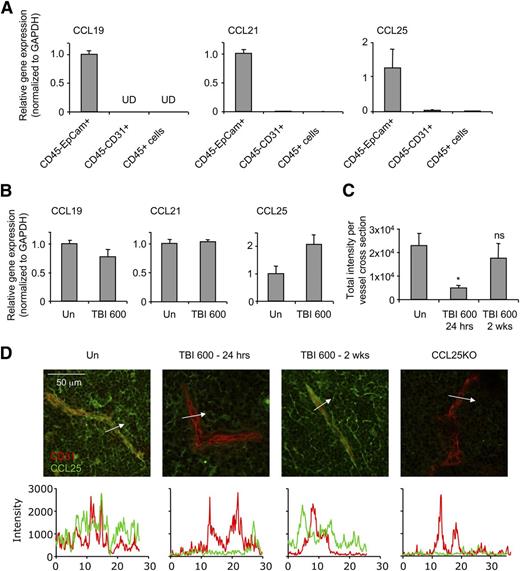
Robust physiologic homing of T-cell progenitors to the thymus relies on CCR9 and CCR7. Their ligands, CCL25 and CCL21/CCL19, respectively, are expressed by TECs.44-46 We isolated TECs (CD45−EpCam+), endothelial cells (CD45−CD31+), and hematopoietic cells (CD45+) from dissociated thymi for quantitative PCR (qPCR) analysis. We found that CCL19, CCL21, and CCL25 mRNA are primarily synthesized by TECs, but these mRNAs were very low or not detected in endothelial or hematopoietic cells (Figure 5A). After 600 cGy irradiation conditioning, surviving TECs continued to express chemokine mRNAs (Figure 5B). These results suggested that even though the few TECs that survive irradiation were still expressing chemokine mRNAs, the greatly reduced numbers of TECs might result in decreased total chemokine available in the thymus.
Chemokine levels on thymic endothelial cells are reduced after irradiation. (A) Unirradiated thymi were sorted into CD45−EpCam+ TECs, CD45−CD31+ endothelial cells, and CD45+ cells (n = 3). Each population was analyzed for CCL19, CCL21, and CCL25 mRNA expression by qPCR and normalized to glyceraldehyde-3-phosphate dehydrogenase. (B) CD45−EpCam+ cells were sorted from unirradiated mice or mice that had received 600 cGy of irradiation 24 hours prior (n = 3). RT-PCR of CCL21, CCL19, and CCL25 expression was normalized to glyceraldehyde-3-phosphate dehydrogenase and quantified relative to the unirradiated control. (C-D) Sections (10 μm) were taken from frozen thymi of untreated WT, TBI 600 cGy (24 hours), TBI 600 cGy (2 weeks), and CCL25-deficient mice and stained with antibodies to CD31 (red) and CCL25 (green). Intensities of cross sections were taken across blood vessels (n = 3). Representative microscopy sections are shown. Plot profile (white arrow) of intensities in each channel was generated in Image J and superimposed. Data are represented as mean ± SEM. *P < .05 compared with unirradiated using the Student t test.
Chemokine levels on thymic endothelial cells are reduced after irradiation. (A) Unirradiated thymi were sorted into CD45−EpCam+ TECs, CD45−CD31+ endothelial cells, and CD45+ cells (n = 3). Each population was analyzed for CCL19, CCL21, and CCL25 mRNA expression by qPCR and normalized to glyceraldehyde-3-phosphate dehydrogenase. (B) CD45−EpCam+ cells were sorted from unirradiated mice or mice that had received 600 cGy of irradiation 24 hours prior (n = 3). RT-PCR of CCL21, CCL19, and CCL25 expression was normalized to glyceraldehyde-3-phosphate dehydrogenase and quantified relative to the unirradiated control. (C-D) Sections (10 μm) were taken from frozen thymi of untreated WT, TBI 600 cGy (24 hours), TBI 600 cGy (2 weeks), and CCL25-deficient mice and stained with antibodies to CD31 (red) and CCL25 (green). Intensities of cross sections were taken across blood vessels (n = 3). Representative microscopy sections are shown. Plot profile (white arrow) of intensities in each channel was generated in Image J and superimposed. Data are represented as mean ± SEM. *P < .05 compared with unirradiated using the Student t test.
Chemokines produced by TECs support the homing of circulating cells into tissues via transcytosis through endothelial cells.47 To examine chemokines expressed on endothelial cells, we costained frozen thymus sections with anti-CD31 (red) and anti-CCL25 (green). Colocalization of CD31 and CCL25 was evident in an unirradiated thymus, whereas CCL25 was absent on the blood vessels of irradiated thymi 4 and 24 hours after irradiation (Figure 5C-D and supplemental Figure 2). Two weeks after irradiation, CCL25 was observed on blood vessels, coinciding with the restoration of efficient homing (supplemental Figure 2 and Figure 3). Together these data suggest that the production of chemokines by the few TECs surviving irradiation may be insufficient to sustain efficient homing in the acute period after irradiation.
Restoration of chemokine signals rescues thymic homing defect
If inadequate chemokines levels underlie the homing impairment after thymic irradiation, we predicted that supplying such chemokines to progenitors ex vivo would restore the defect. To restore chemokine signals to the trafficking progenitors, we pretreated BM cells with CCL21 and CCL25 at 37°C for 30 minutes. Untreated or pretreated BM was injected into irradiated recipients. We detected homing of pretreated BM within 44 hours to irradiated thymi in 5 of 6 mice (Figure 6A). We detected CCL21/CCL25-mediated homing to the irradiated thymus as early as 4 hours after injection of donor cells (supplemental Figure 3). To determine whether chemokine stimulation was increasing the proliferation or survival of progenitors, freshly isolated BM progenitors were incubated with CCL21 and CCL25 and cultured on OP9-DL4 stromal cells. After 10 days of culture, untreated and treated progenitors gave rise to similar numbers of Thy1+CD25+ cells (Figure 6B). Together, these results support the interpretation that pretreatment of progenitors with appropriate chemokine ligands did not affect their survival or proliferation but instead allowed their migration into the thymus.
Pretreatment with CCL21/CCL25 rescues homing to the thymus after irradiation. (A) BM was incubated with PBS or CCL21 and CCL25 for 30 minutes at 37°C. Pretreated BM cells (107) were injected into unirradiated recipients or mice that had received 600 cGy TBI for the short-term homing assay (n = 3). Representative flow cytometric plots of cocultures are shown. P < .05 using Fisher’s exact test. (B) One hundred sorted Flt3+LK cells pretreated with CCL21 and CCL25 for 30 minutes were grown on OP9D4 in the presence of IL7 and Flt3. Cocultures were analyzed by flow cytometry after 10 days. Number of Thy1+CD25+ cells are shown. (C) BM was incubated with blocking anti-α4 prior to pretreatment or CCL21/CCL25 was washed from the cells following pretreatment and left for 30 minutes at 37°C. After 14 days, thymi were examined for donor reconstitution by flow cytometry (n = 3). (D) After 16 days, BM and thymi from PBS or pretreated groups were examined for total thymocyte numbers and donor reconstitution by flow cytometry (n = 3). Data are represented as mean ± SEM. *P < .05 compared with untreated using the Student t test.
Pretreatment with CCL21/CCL25 rescues homing to the thymus after irradiation. (A) BM was incubated with PBS or CCL21 and CCL25 for 30 minutes at 37°C. Pretreated BM cells (107) were injected into unirradiated recipients or mice that had received 600 cGy TBI for the short-term homing assay (n = 3). Representative flow cytometric plots of cocultures are shown. P < .05 using Fisher’s exact test. (B) One hundred sorted Flt3+LK cells pretreated with CCL21 and CCL25 for 30 minutes were grown on OP9D4 in the presence of IL7 and Flt3. Cocultures were analyzed by flow cytometry after 10 days. Number of Thy1+CD25+ cells are shown. (C) BM was incubated with blocking anti-α4 prior to pretreatment or CCL21/CCL25 was washed from the cells following pretreatment and left for 30 minutes at 37°C. After 14 days, thymi were examined for donor reconstitution by flow cytometry (n = 3). (D) After 16 days, BM and thymi from PBS or pretreated groups were examined for total thymocyte numbers and donor reconstitution by flow cytometry (n = 3). Data are represented as mean ± SEM. *P < .05 compared with untreated using the Student t test.
To determine whether an increase in early homing by chemokine-treated BM enhances thymic reconstitution, untreated or pretreated BM was injected into irradiated recipients, and the thymus and BM were examined after 14 or 16 days. At 14 days, donor engraftment in the thymus was increased in recipients that received pretreated BM, suggesting that signals provided by CCL21 and CCL25 improves thymic reconstitution through increased homing of progenitors (Figure 6C). To determine whether chemokine treatment of progenitors could be bypassing other requirements for homing, we incubated BM cells with a blocking antibody against α4 integrin prior to chemokine pretreatment; α4 integrin blockade prevented the increase in donor engraftment. Removal of chemokines from lymphocytes returns integrins to their preactivation conformation.48 We therefore assessed thymic reconstitution of pretreated cells that were removed from chemokines for 30 minutes prior to intravenous injection. This also inhibited the CCL21/CCL25-mediated increase in donor engraftment (Figure 6C). At 14 days after irradiation, the thymus is largely comprised of host-derived cells that survived the irradiation and proliferated; however, 16 days after irradiation, the thymus becomes almost equivalently donor and host. At this time, both donor-derived thymocyte numbers and total thymic cellularity were significantly increased by pretreatment with CCL21 and CCL25 (Figure 6D). Interestingly, improved donor settling in the thymus increased the numbers of host thymocyte populations (data not shown), similar to previous observations.49,50 The facilitated reconstitution of host-derived cells may be through the restoration of thymic architecture due to lymphocyte-stromal interactions.50 As expected, LSK engraftment in the BM was equivalent between untreated and pretreated cells (Figure 6D), consistent with the absence of CCR7 and CCR9 on HSCs and MPPs. Another chemokine receptor, CXCR4, although insufficient for efficient thymic homing physiologically, is broadly expressed on hematopoietic progenitor populations.13 To determine whether signaling via any chemokine receptor expressed by progenitors is sufficient to restore the defect after irradiation, we pretreated BM with the CXCR4 ligand CXCL12. Pretreatment with CXCL12 improved thymic reconstitution similar to what was seen with CCL25 and CCL21, but did not impact BM reconstitution by LSK cells (supplemental Figure 4). This suggests that chemokine-induced activation of progenitors does not necessarily require the physiologically relevant chemokines. CXCR4, CCR7, and CCR9 are all coupled to Gαi, and this shared mechanism would suggest that the consequences for progenitors would therefore be qualitatively similar whether signals were provided via CXCR4, CCR9, or CCR7. Together, these data demonstrate that pretreatment of BM progenitors with chemokine signals improves thymic reconstitution, presumably by increasing the efficiency of thymic settling.
Discussion
Here we studied homing of progenitors to the thymus after conditioning regimens for BMT. Our results reveal that progenitor cell homing becomes undetectable after BMT conditioning, that homing is limited by chemokine signals, and that exogenous treatment with chemokines restores thymic homing. Thymic settling by progenitors has been difficult to study, likely because this event is exceedingly rare.51 We report here that, under physiologic conditions, very few intravenously injected lymphoid progenitors settle the thymus, highlighting the inefficiency of this process. The reasons for the low frequency of circulating progenitors that home to the normal thymus are still unclear. Several nonexclusive possibilities exist; among them are the following: (1) there may be additional homing molecules required, which would further restrict trafficking to the thymus within these populations; and (2) other tissues trap or attract progenitors of T cells, reducing the supply available to the thymus.
The identity of thymic-homing progenitors has been controversial, and LMPPs and/or CLPs have each been proposed to directly settle the thymus.25,26 We demonstrate that both LMPPs and CLPs, but not earlier progenitors, can home to the thymus when intravenously injected into normal unconditioned mice. These demonstrated abilities of LMPPs and CLPs align with the expression of CCR9 and CCR7 by these progenitor populations52 and also with their expression of functional PSGL-1.19
After BMT, homing of progenitors to the thymus was greatly reduced. Progenitors trafficking from the blood to the thymus are thought to use mechanisms analogous to those used by mature lymphocytes to enter lymph nodes in which selectin-mediated rolling is followed by chemokine signals activating integrins on the surface of trafficking cells. The activation mediates firm binding with adhesion molecules on endothelial cells, leading to arrest of the rolling cell. The cells are then able to transmigrate from the vasculature into the organs.20,53 Our data demonstrate that the thymus becomes unreceptive to progenitor trafficking transiently after irradiation. Endothelial cell numbers and endothelial cell expression of selectins and integrins was unaffected; however, irradiation resulted in the death of chemokine-producing TECs, likely reducing chemokine availability to progenitors circulating through the thymic vasculature.
Providing exogenous chemokine ligands prior to intravenous injection restored thymic settling of progenitors into irradiated thymi, confirming that chemokine signals are limiting after irradiation. These data also indicate that a localized chemokine signal or gradient is not strictly necessary for homing to the thymus. Homing was not rescued in all of the recipients, which may be due to either additional damage caused by irradiation, which we have not explored here, or may indicate that the activation of the cells ex vivo is less efficient than physiologic activation. CCL21 and CCL25 signaling through their respective receptors have been shown to convert integrins to their high affinity conformations, which mediate firm adhesion of lymphocytes to the endothelium.48,54 The integrins VCAM-1 and LFA-1 on lymphocytes are activated by chemokines; however, chemokine stimulation of LFA-1 is transient; the cell adhesion to ICAM-1 lasts on the order of minutes.48 These data suggest that any chemokine-induced ICAM-1/LFA-1-dependent progenitor adhesion to the thymic endothelium likely occurs within a few minutes of intravenous injection. The active conformation of VLA-4 may be slower to revert and may used by intravenously injected progenitors to bind to VCAM-1 on thymic endothelial cells.
Our investigations of thymic reconstitution were performed in a syngeneic transplant model with young mice, which presents several caveats. Our results may not be fully representative of thymic reconstitution in aged humans who have involuted thymi. Our model also avoided any complications that graft-versus-host disease may have caused. In young mice, we demonstrate that a small number of LMPPs and CLPs traffic to the thymus, and this amount of homing is further limited by chemokine signals following irradiation. In young healthy mice, homing recovered at least partially after 2 weeks, but whether similar recovery occurs in all humans awaits further studies. However, our results suggest 2 possible methods for enhancing T-cell precursor trafficking to the thymus. One method is to administer a second inoculum containing progenitors (LMPPs and/or CLPs) after a period when CCR7/CCR9-dependent efficient thymic homing is re-established in humans. The second method is pretreatment of BM progenitors with chemokines prior to transfer into patients. Both may improve T-lineage reconstitution following BMT. These approaches could serve as useful adjuncts to administration of keratinocyte growth factor, previously shown to increase TEC numbers, and cytokines such as Flt3 ligand, which targets hematopoietic cells.55,56 Improving trafficking of progenitors to the thymus alongside therapies to ameliorate graft-versus-host disease and thymic involution may together enhance T-cell regeneration following BMT.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
The authors thank Mark I. Greene and David B. Roth of the Department of Pathology and Laboratory Medicine for support and Christelle Harly, Drew Comrie, Skye Geherin, Gudrun Debes, and Jan Burkhardt for helpful discussions. The authors also thank Tianying Jiang and the Abramson Cancer Center Histology Core for technical support.
This work was supported by National Heart, Lung, and Blood Institute grant R01HL11074103 (to A.B.) and National Cancer Institute grant P01CA065493 (to B.R.B.) of the National Institutes of Health.
Authorship
Contribution: S.L.Z. performed most of the experimental work; S.M., D.A.Z., X.W., and J.L.B. contributed to the performance of experiments; S.L.Z. and A.B. planned the project; S.L.Z., X.W., and A.B. analyzed data and prepared the manuscript; and B.R.B. discussed experiments and data.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Avinash Bhandoola, 277 John Morgan Building, 3620 Hamilton Walk, Philadelphia, PA 19104; e-mail: bhandooa@mail.med.upenn.edu.