Abstract
INTRODUCTION AND AIMS
Almost seventy percent of Acute Myeloid Leukemia (AML) patients will relapse. Fludarabine, Ara-C, Idarrubicine, G-CSF (FLAG-Ida) and FLAG-Ida and Gentuzumab-Ozogamicin (FLAGO-Ida) is frequently used before allogenic stem cell transplant (ASCT). There are several studies analyzing the risk factors for survival in relapsed/refractory patients, but none of them were performed exclusively in patients treated with FLAG-Ida. We analyzed the results of in the Spanish trials conducted by PETHEMA. We also present a predictive score system of survival prognosis based on the data of the patients enrolled
METHODS
This is a retrospective, multicentre study of the PETHEMA AML epidemiologic Registry. In PETHEMA protocols 98,99,2007,2010 and 2011, FLAG-Ida was included as salvage treatment in patients relapsed or resistant after a 2ndinduction based in 3+7. In PETHEMA trial 2007, FLAG-Ida (or FLAGO-Ida) was administered if complete remission (CR) was not attained in the first induction cycle. The use of FLGO in PETHEMA 2007 trial depended on the availability of GO for each centre.
We use overall survival (OS) as final end point of the analysis. Univariate survival analysis was performed by Long-Rank test. Stepwise backward variable selection and Cox proportional hazard multivariable analysis was performed (covariables selected if p<0.01). Bootstrap analysis was performed to contrast the model. We stratified time to therapy (TTT) in three stratus: refractory, relapsed in more than 1 year or less than 1 year. Refined Medical Research Council (MRC) cytogenetic risk stratification was used (patients with relapsed t(8;21) had the same OS as adverse group).
RESULTS
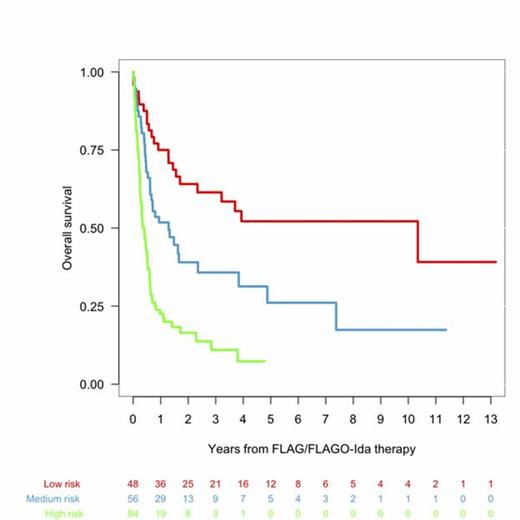
We analyzed 259 patients consecutively registered between 1999 and 2012. The median age was 52 (16-76) and the median follow-up of the patients still alive was 23 months. Only 38 (14%) patients received FLAGO-Ida as salvage treatment. Favorable karyotype (inv 16) and t(8;21) was present in 13 (6%) and 11 (5%) respectively; intermediate in 145 (62%) and adverse in 62 (21%). Previous ASCT was performed in 86 patients (33%). The number of patients who achieved CR or CR without recovery of peripheral counts (CRi) was 132 (52%), PR in 23 (9%). Resistance accounted for 75 (29%) and death for 24 (9%). For the purposes of the current study, we decided to include patients with t(8;21) in the group of adverse karyotype group due to the similar median OS compared with MRC adverse karyotype (Median OS,0,42 vs 0,45 years). In univariate analysis karytotype (p=0.0001), FLT3-ITD positive (p=0.005), previous transplant (comparing no transplant, autologous and allogenic, p=0.012) and time to relapse (comparing refractory vs <12 months vs >12 months; p=0.0002) were significant risk factors. Contrary to other studies previous ASCT was associated to better outcome (median OS=2.3years, p=0.012). In multivariate analyses, the following variables remained as independent risk factors for survival: TTT (p=0.003), cytogenetic risk (p<0.00001), FLT3 (p=0.001), previous SCT (p=0.003). A simplified scoring system for survival in patients receiving FLAG-Ida or FLAGO-Ida was raised using the significant variables: cytogenetics (favorable: 0 points, intermediate 2 points, adverse 4 points), previous SCT (ASCT: 0, no SCT: 1, auto: 2), TTT (relapse>12 months: 0, refractory: 2, relapsed<12 months: 4,) and FLT3-ITD (wild type=0, mutated=2 points,). We stratified into three groups: Low-risk (0-4 points, 27% of patients, median OS 10.3 years), Intermediate-risk (5-8 points, 30% of patients, median OS 1.3 years), and high-risk (>8 points, 44.6% of patients, median OS 0.4 years).
CONCLUSIONS: This retrospective study based on an Epidemiologic Registry, shows that in primary refractory and first relapsed AML patients, the use FLAG-Ida or FLAGO-Ida salvage regimens lead to 52% of second CR. In addition, we have developed a scoring system using FLT3-ITD, refined cytogenetics, previous ASCT and TTT that can predict survival, identifying before administering FLAG-IDA a good prognosis population representing 27% of patients in which FLAG-IDA (followed by ASCT) can be an optimal therapy. This prognostic index should be validated in an independent cohort.
No relevant conflicts of interest to declare.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal