Abstract
Background: Cord blood (CB) is routinely used as an alternative stem cell source for the treatment of children with acute leukemia and has been associated with high rates of disease-free survival (DFS). However, one controversial issue in pediatric CB transplantation is the role of anti-thymocyte globulin (ATG) as immunoprophylaxis. While inclusion of ATG in the conditioning may decrease the risk of graft-versus-host disease (GVHD), it could potentially abrogate graft-versus-leukemia effects and increase the risk of opportunistic infections. Thus, we compared outcomes in a uniform group of pediatric patients with acute lymphoblastic leukemia (ALL) who underwent myeloablative CB transplantation with or without ATG.
Methods: Patients with ALL in morphologic remission (CR1 n=106, CR2 n=146, CR3 n=45) aged ≤ 20 years transplanted between 01/2007-12/2011 with high-dose total body irradiation-based conditioning and single or double unit CB grafts were eligible for analysis. CB units were 4-6/6 HLA-A,-B at the antigen-level, -DRB1 allele matched to the recipient and had CB unit with cryopreserved total nucleated cell dose of ≥ 2.5 x107/kg/unit. Cox regression models were built to evaluate potential differences in outcome in ATG versus non-ATG CB transplant recipients. The primary endpoint was 3-year DFS.
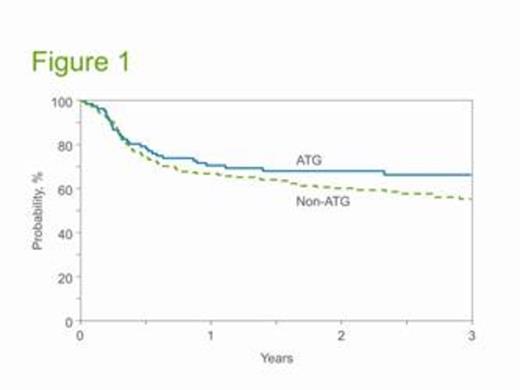
Results: Of 297 patients, 92 received ATG and 205 did not. Age and disease status were similar in each group whereas ATG recipients were less likely to be CMV seropositive and more likely to receive single unit CB grafts. While neutrophil engraftment was similar in each group, the risk of day 100 grade II-IV acute GVHD [30% (95%CI: 21-40) versus 54% (95%CI: 47-61), p = 0.0002] and 3-year chronic GVHD [22% (95% CI: 14-31) versus 43% (95% CI: 36-50), p = 0.0008] were decreased in ATG recipients. However, day 100 grade III-IV aGVHD was comparable: 11% (95%CI: 5-18) in ATG versus 17% (95%CI: 12-23) in non-ATG recipients, p = 0.15]. The 3-year TRM was similar in both groups: 16% (95%CI: 10-25) in ATG versus 17% (95%CI: 13-23) in non-ATG recipients (p = 0.98). Relapse was also similar in ATG and non-ATG recipients: 17% (95%CI: 10-23) versus 27% (95%CI: 21-34, p = 0.12), respectively. In multivariate analysis, negative CMV serostatus was associated with reduced TRM risk [HR 0.55 (95%CI: 0.30-0.98), p = 0.004] whereas remission status CR2 or CR3 significantly increased relapse risk [HR 2.18 (95%CI: 1.22-3.89), p = 0.008], but inclusion of ATG had no effect on either outcome. With a median follow-up of survivors of 36 months (range 5-72), the 3-year DFS was 66% (95%CI: 56-76) and 55% (95%CI: 48-62) in ATG and non-ATG recipients, respectively (p = 0.23, Figure 1). The distribution of causes of death was similar in each group. In multivariate analysis, treatment failure risk was increased in patients transplanted in CR2 or CR3, but the inclusion of ATG had no effect (p = 0.24) (Table 1).
Conclusion: Inclusion of ATG in pediatric myeloablative CB transplant for ALL is associated with a decreased risk of grade II-IV acute GVHD and chronic GVHD but not severe grade III-IV acute GVHD. There was no difference in 3-year DFS in each group and multivariate analysis revealed ATG inclusion had no impact upon treatment failure risk. These results indicate that optimization of both ATG and non-ATG conditioning platforms are needed in order to further improve CB transplantation survival in children with ALL. Unanswered questions include the impact of the formulation, dose and timing of ATG administration. These findings cannot be extrapolated for other diagnoses, reduced intensity conditioning, or adults.
| . | Hazard ratio (95% confidence interval) . | p-value . |
|---|---|---|
| ATG use Conditioning without ATG Conditioning with ATG | 1.00 0.78 (0.52 – 1.18) | 0.24 |
| Disease status CR1 CR2, CR3 | 1.00 2.01 (1.31 – 3.08) | 0.001 |
| . | Hazard ratio (95% confidence interval) . | p-value . |
|---|---|---|
| ATG use Conditioning without ATG Conditioning with ATG | 1.00 0.78 (0.52 – 1.18) | 0.24 |
| Disease status CR1 CR2, CR3 | 1.00 2.01 (1.31 – 3.08) | 0.001 |
No relevant conflicts of interest to declare.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal