Abstract
A relationship between the microbiota and GVHD has long been suspected but is still not well understood. Recently, several studies have indicated that obligately anaerobic commensal intestinal bacteria may be beneficial for intestinal health. Recent studies have found that obligate anaerobes in the intestine, in particular Clostridial species, are important mediators of intestinal homeostasis and prevent inflammation by upregulating intestinal regulatory T cells (Tregs). In this study we sought to further characterize the relationship between the microbiota and GVHD, focusing on the effects of antibiotics (ABX) with anaerobic coverage.
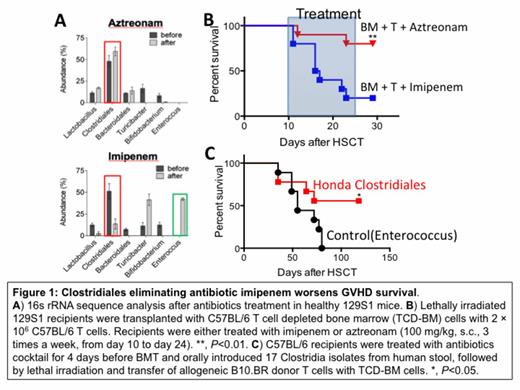
First, we treated healthy 129S1 mice with either ABX that included significant activity against anaerobic bacteria (piperacillin-tazobactam; pip-tazo, imipenem-cilastatin; imipenem or ones with reduced activity (cefepime, aztreonam). Mice were treated by subcutaneous injections of each antibiotic twice a day for two days (500 mg/kg for pip-tazo and 100 mg/kg for others) and stool samples were collected, followed by 16S rRNA gene amplification and sequence analysis. We found that treatment with pip-tazo and imipenem significantly reduced the abundance of anaerobic Clostridial species and increased that of Enterococcus, while treatment with cefepime and aztreonam spared Clostridiales (Figure 1A). We next investigated the effects of antibiotic treatment in a clinically relevant MHC matched, minor antigen mismatched murine allogeneic BMT model. Lethally irradiated 129S1 recipients were transplanted with C57BL/6 T cell depleted bone marrow cells and 2 × 106 C57BL/6 T cells. Recipients were either treated with imipenem or aztreonam subcutaneously beginning on day +10 after BMT. We observed significantly worsened GVHD survival in imipenem-treated recipients (Figure 1B, P<0.01). To further explore the impact of anaerobic bacteria on GVHD, we treated recipient mice with an oral combination of ampicillin, clindamycin, vancomycin and metronidazole and thereafter re-introduced a mixture of 17 Clostridia isolates derived from human stool previously shown to upregulate Tregs in the colon of mice. We found that introduction of this Clostridial mixture to mice before BMT resulted in improved GVHD survival (Figure 1C, P<0.05). Finally, we evaluated the impact of ABX treatment on graft-versus-tumor (GVT) activity and found that elimination of gut bacteria with pip-tazo did not alter GVT activity in mouse A20-TGL tumor BMT model. Together, these results suggested that selection of ABX that spare anaerobic bacteria may be an effective strategy to reduce GVHD without impacting on GVT, and that this effect may be mediated by maintaining Clostridial commensals.
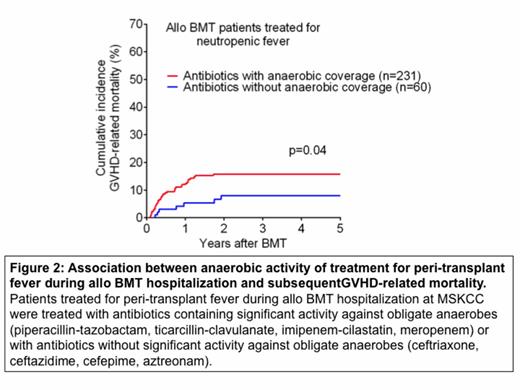
We have also retrospectively evaluated the impact of antibiotic spectrum of activity on clinical GVHD-related mortality. We examined a cohort of 546 adult patients transplanted at MSKCC from 1992 to 2013 who received conventional (non-T cell depleted) grafts and received treatment for peri-transplant fever with empiric ABX classified as either including anaerobic coverage or with reduced anaerobic activity. Of these, 156 patients who received ABX from both classifications were excluded, as were 99 patients exposed to other ABX with anaerobic coverage not routinely used to treat peri-transplant fever. We examined the impact of anaerobic coverage in the remaining 291 patients. Therapies for peri-transplant fever were divided into those that included significant activity against anaerobic bacteria (pip-tazo, ticarcillin-clavulanate, imipenem, meropenem), and those with reduced activity (ceftriaxone, ceftazidime, cefepime, aztreonam). Additional ABX that have anaerobic activity but were not routinely used to treat peri-transplant fever included metronidazole, oral vancomycin and clindamycin. We found that 60 patients that received ABX without anaerobic coverage had a significantly reduced incidence of GVHD-related mortality, compared to 231 patients treated with anaerobe-active ABX (Figure 2, P<0.05). These results raised the possibility that anaerobic commensals may be protective against GVHD in humans as well as in murine models.
Taken together, our results suggest that a population of anaerobic commensals may mediate protection against life-threatening GVHD and that selecting ABX with a more limited spectrum of activity can prevent microbiota injury and reduce GVHD.
No relevant conflicts of interest to declare.
Author notes
Asterisk with author names denotes non-ASH members.
This icon denotes a clinically relevant abstract

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal