Abstract
Background. Array-based technology has been showing a great impact on clinical cancer cytogenetic, especially on genetically heterogeneous disease, such as MM, where relevant lesions might be the hallmarks of different patients’ subgroups, thus becoming of clinical relevance as well.
We present herein the results of a molecular sub-study of the EMN02 phase III study (EMN02_HOVON95) which was designed to compare consolidation therapy Bortezomib, Melphalan and Prednisone versus upfront autologous stem cell transplantation, both applied after induction therapy with bortezomib-cyclophosphamide-dexamethasone (VCD).
The sub-study was aimed at developing a comprehensive, high throughput genomic profile to be used to stratify uniformly treated MM patients according to their genomic background at baseline and to perform correlations with response to induction therapy.
Patients and methods. Data obtained from 170 patients who consecutively entered the study and received three 21-day cycles of VCD induction therapy were analyzed. Baseline patients’ characteristics, including cytogenetic abnormalities, were comparable with those of 717 patients enrolled by participating Italian centres. Highly purified CD138+ bone marrow plasma cells were profiled by SNPs array (Affymetrix 6.0 and CytoScanHD® chip). ChAS (Affymetrix) and Nexus Copy NumberTM 7.5 (Biodiscovery) software were used to perform Copy Number Alterations (CNAs) analyses and clinical correlations, respectively.
Results. After induction therapy, 66 out of 170 (38.8%) patients achieved a very good partial response (VGPR) or better, including 15 (8,8%) who attained a complete response (CR). On the contrary, 104/170 (61.1%) patients achieved <=partial response (PR), including 28 with stable disease (SD).
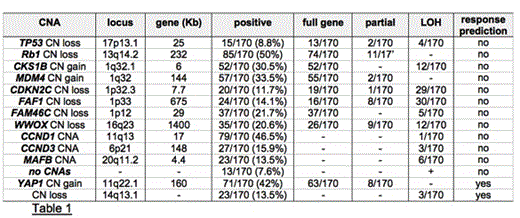
Presenting MM cases were studied by SNPs array in order to compute CNAs and acquired loss of heterozygosity (LOH) in the tumor. The frequency distribution of the more relevant CNAs is summarized in table 1. A subgroup of 13/170 (7.6%) patients was characterized by the absence of any macro CNAs (either gains or losses): these cases were mainly characterized by LOH events on chr. 1, 8 and 16, where putative tumor suppressor genes are located (e.g. PLEKOH1 and SIAH1 on chr.1 and 16, respectively). In order to identify novel chromosomal lesions potentially influencing response to induction therapy, we compared the CNAs profile of the extreme response categories, i.e. CR and SD. Neither the absence of CNAs nor the presences of any of those that are prognostically relevant were significantly linked to response to induction therapy. On the contrary, the following two novel lesions resulted highly significant. A 42.9 Kb CN gain on chr.11q22.1-22.2, which only includes the Hippo pathway mediator YAP1, significantly characterized 6% of patients in CR, as compared to 54% of patients with SD (p=0.002). An extended CN loss on chr.14q13.1-13.3, including genes implicated in the progression on cancer (e.g. NKX2-8), significantly characterized 62.5% of patients who achieved CR, as compared to 4% of those with SD (p<0.001).
Conclusions. The reconstruction of high-throughput virtual karyotype by SNPs array in a cohort of homogeneously treated, newly diagnosed, MM patients offered the opportunity to obtain a comprehensive overlook of each patient’s sub-chromosomal anatomy. This allowed both to perform a detailed patients’ stratification at diagnosis and to identify, among the whole spectrum of CNAs, those having an impact on response to induction therapy.
Palumbo:Bristol-Myers Squibb: Consultancy, Honoraria; Genmab A/S: Consultancy, Honoraria; Celgene: Consultancy, Honoraria; Janssen-Cilag: Consultancy, Honoraria; Millennium Pharmaceuticals, Inc.: Consultancy, Honoraria; Onyx Pharmaceuticals: Consultancy, Honoraria; Array BioPharma: Honoraria; Amgen: Consultancy, Honoraria; Sanofi: Honoraria. Sonneveld:Celgene: Research Funding, Speakers Bureau; Millennium-Takeda: Research Funding; Onyx: Research Funding, Speakers Bureau; Janssen: Speakers Bureau. Cavo:Millenium: Consultancy, Honoraria; BMS: Consultancy, Honoraria; Celgene: Consultancy, Speakers Bureau; Janssen: Consultancy, Speakers Bureau; Onyx: Honoraria.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal