Abstract
Introduction: The incidence of subsequent primary malignancies (SPM) associated with lenalidomide treatment of multiple myeloma (MM) outside the context of maintenance therapy post-melphalan is unknown. Three clinical trials reported modest, statistically significant increased risks of SPM associated with lenalidomide treatment in MM patients (Palumbo et al, N Engl J Med, 2012; Attal et al, N Engl J Med, 2012; McCarthy et al, N Engl J Med, 2012). Although these randomized trials are well controlled for potential confounders, they represent a unique population of patients and a specific juxtaposition of lenalidomide use with melphalan; as such, their results are not necessarily generalizable to the broader MM patient population. To investigate whether lenalidomide is associated with an increased risk of SPM in MM patients within a clinical setting in the United States, we conducted a retrospective cohort study of 1,653 MM patients treated with or without lenalidomide at the Moffitt Cancer Center (MCC) in Tampa, FL.
Methods: Patients treated for MM at MCC from 2004-2012 were identified through Moffitt's enterprise wide data warehouse combining clinical information from several sources, including the Cancer Registry, electronic medical records and disease-specific databases. Among 1,653 MM patients, ages 18 and older, 51 cases of SPM were verified by two hematologists for confirmation of MM and SPM diagnoses. Incidence rates and 95% confidence intervals (CI) for SPM were estimated using a Poisson distribution. Cox proportional hazards ratios (HR) and 95% CIs were calculated to estimate the age-adjusted association between lenalidomide treatment and SPM in the overall cohort, and stratified by ISS. Additional details on lenalidomide treatment and potential confounders were obtained through medical chart abstraction for SPM cases and a subset of MM patients from the baseline cohort who had not developed SPM; these controls were matched to cases 2:1 on age at MM diagnosis (+/- 5 years), gender, follow-up time (+/- 6 months), and date of diagnosis (+/- 1 year). Associations between lenalidomide and SPM in the nested case-control analysis were estimated using odds ratios (OR) and 95% CIs adjusted for age at MM diagnosis, bone marrow transplantation, creatinine levels and personal history of cancer.
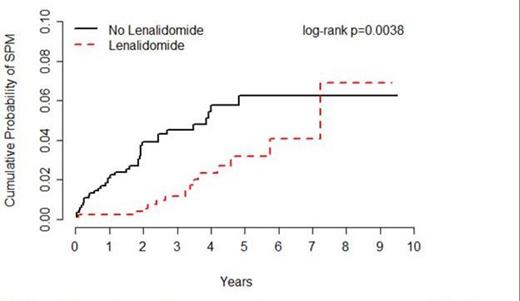
Results: Overall, 1,653 MM patients were followed for an average of 40 months, including patients treated with (n=846) or without (n=807) lenalidomide. Incident SPMs were observed for 15 patients treated with lenalidomide (0.55 per 100 person-years) and 36 patients treated without lenalidomide (1.27 per 100 person-years), corresponding to an HR of 0.44 (95% CI=0.24-0.80) (Figure 1). Of the 51 SPMs observed, 37 were solid tumors comprising 14 different types, including 9 and 28 in the lenalidomide and no lenalidomide groups, respectively (HR=0.55, 95% CI=0.15-0.69). Of the 14 hematological SPMs observed, 8 were in the lenalidomide group versus 6 in the no lenalidomide group (HR=0.90, 95%CI = 0.31-2.63). Similar associations between lenalidomide and SPM were observed for MM patients with ISS = 1 (HR=0.26, 95% CI=0.06-1.21) and for MM patients with ISS = 2 or 3 (HR=0.20, 95% CI=0.02-1.62). Of the 51 SPM cases and 102 matched controls included in the nested case-control analysis, 33.3% and 74.5% were treated with lenalidomide, respectively (adjusted OR=0.03, 95% CI=0.002-0.34). Similar associations were observed for lenalidomide given as part of first line treatment versus subsequent treatment, and for lenalidomide given alone or in combination with other drugs. (8 cases and 46 controls received melphalan in addition to lenalidomide.) There was no association between lenalidomide and SPM among those treated for >9.1 months (OR=0.05, 95% CI=0.01-0.43), the median treatment duration among controls.
Conclusion: Lenalidomide treatment was not associated with an increased risk of SPM among a large cohort of MM patients. It is important to note that in this clinical setting (in 2004-2012) the use of lenalidomide in combination with melphalan and in the maintenance setting was a rare event. This may be a critical factor in the contrast between the results of this study and in the increase in SPMs reported in randomized clinical trials.
Incidence of SPM among patients treated for MM with or without lenalidomide, Moffitt Cancer Center, 2004-2012
Incidence of SPM among patients treated for MM with or without lenalidomide, Moffitt Cancer Center, 2004-2012
Rollison:Celgene, Inc.: Research Funding. Off Label Use: Lenalidomide given as treatment for non-del(5q) MDS and/or multiple myeloma . Komrokji:Celgene: Consultancy, Research Funding. Lee:Celgene, Inc.: Research Funding. Hampras:Celgene, Inc: Research Funding. Fulp:Celgene, Inc.: Research Funding. Fisher:Celgene, Inc: Research Funding. Baz:Celgene: Research Funding; BMS: Research Funding; Millenium: Research Funding; Sanofi: Research Funding; Karyopharm: Research Funding. Olesnyckyj:Celgene: Employment, stock options Other. Kenvin:Celgene: Employment, stock options Other. Knight:Celgene, Inc: Employment, stock options Other. Dalton:Celgene, Inc.: Research Funding; Novartis: Consultancy, Honoraria; Genentech: Consultancy, Honoraria. Shain:L&M Healthcare/Onyx/Amgen: Research Funding, Speakers Bureau; Envision/Celgene: Research Funding, Speakers Bureau.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal