Abstract
Introduction: Blinatumomab, an investigational bispecific T-cell engager (BiTE®) antibody construct, has been shown to induce remission in adult patients with relapsed/refractory BCP-ALL. Medically important adverse events (AEs) related to blinatumomab treatment in adults are cytokine release syndrome (CRS) and neurological events. We report the primary analysis of the phase 1 portion of a multicenter phase 1/2 study of blinatumomab in pediatric patients with relapsed/refractory BCP-ALL.
Methods: In this continuing study, eligible patients are <18 years old and must have BCP-ALL that is in second or later bone marrow relapse, in any marrow relapse after allogeneic hematopoietic stem cell transplantation (HSCT), or refractory to induction or reinduction therapy. Patients receive blinatumomab for 28 days by continuous intravenous infusion followed by a 14-day treatment-free period (for up to five cycles). Escalating dosing levels of 5, 15, and 30 μg/m²/day and stepwise dosing of 5–15 or 15–30 μg/m²/day were evaluated. The primary endpoint of the phase 1 portion of the study was maximum tolerated dose (MTD). Secondary endpoints included toxicity, complete remission (CR) rate, overall survival (OS), relapse-free survival (RFS), pharmacokinetics evaluation, and cytokine measurement.
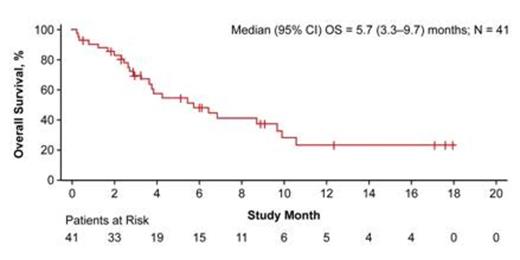
Results: In the phase 1 portion, 41 patients received a total of 73 cycles (median of 2 cycles received, range of 1 to 5). Eight (20%) patients had refractory disease and seven (17%) had experienced at least two bone marrow relapses. Twenty-six (63%) patients had relapsed following HSCT. Dose-limiting toxicities (DLTs) are listed in Table 1. The MTD was established at 15 µg/m²/day. To decrease the risk of CRS, a stepwise dose of 5–15 μg/m²/day was recommended for the phase 2 part of the study (5 µg/m²/day for 7 days, then 15 µg/m²/day). This dose was subsequently assessed in two age groups (2–6 and 7–17 years) in the phase 1 expansion part with one of 18 patients developing grade 3 CRS. No patient in the expansion cohort developed grade 4 or 5 CRS. Across all dosing levels, 13 (32%) patients had CR with 10 (77%) achieving minimal residual disease (MRD) negativity. Of these 13 patients, nine (69%) underwent HSCT. Among patients who achieved CR, median RFS was 8.3 months (95% CI: 3.0–16.0 months). Median OS was 5.7 months (95% CI: 3.3–9.7 months; Figure 1) with a median follow-up time of 12.4 months. Across all dosing levels, the most common AEs regardless of causality were pyrexia (78%), headache (37%), hypertension (32%), nausea (29%), abdominal pain (27%), pain in the extremity (27%), and anemia (27%). Pharmacokinetic parameters, including steady-state concentration (Css), clearance, and half-life were similar to those from adult patients with relapsed/refractory BCP-ALL who received body surface area-based blinatumomab dosing. Transient elevations of serum cytokines were observed mostly within the first two days after starting blinatumomab, in particular IL-6, IFN-gamma, IL-10, and, to a lesser extent, IL-2 and TNF-α.
Conclusions: In the phase 1 portion of this study in pediatric patients with relapsed/refractory BCP-ALL, the MTD was 15 µg/m²/day. CRS was dose-limiting, but stepwise dosing of 5–15 μg/m²/day has been effective in ameliorating CRS. Thirty-two percent of patients achieved CR and more than half were able to proceed to allogeneic HSCT.
von Stackelberg:Amgen: Consultancy, Honoraria. Off Label Use: This presentation will discuss the off-label use of blinatumomab, as this agent is not approved for use by the FDA, EMA or any other regulatory authorities.. Zugmaier:Amgen Inc.: Equity Ownership; Amgen Research (Munich) GmbH: Employment. Rheingold:Novartis: Consultancy. Hu:Amgen Inc.: Employment, Equity Ownership. Mergen:Amgen Inc.: Equity Ownership; Amgen Research (Munich) GmbH: Employment. Fischer:Amgen Inc.: Equity Ownership; Amgen Research (Munich) GmbH: Employment. Zhu:Amgen Inc.: Employment, Equity Ownership. Hijazi:Amgen Inc.: Equity Ownership; Amgen Research (Munich) GmbH: Employment. Gore:Amgen Inc.: Travel Support Other.
Author notes
Asterisk with author names denotes non-ASH members.


This feature is available to Subscribers Only
Sign In or Create an Account Close Modal