Abstract
Background:
Allogeneic bone marrow transplantation for patients with hematological malignancies is curative, but the success of the procedure is limited by graft versus host disease (GvHD). Donor T-cells present in the graft are critical for the graft-versus-leukemia effect (GvL) of the transplant and important in facilitating bone marrow reconstitution, but also contribute to development of GvHD, and the risk of GvHD increases dramatically when the donor and recipient are not matched at all of the HLA loci. Hence, there is an urgent need for new approaches to curb GvHD and facilitate transplantation of HLA mismatched grafts to improve patient outcomes. Here, we have used an MHC-mismatched mouse model of GvHD to develop a novel and powerful approach to improve the success of allogeneic bone marrow transplantation by limiting GvHD. We identified a tryptophan metabolite, indole-3-carboxaldehyde (ICA), a dietary component present in foods such as collard greens and broccoli, that prevents lethal GvHD and allows normal engraftment by allogeneic donor bone marrow when fed to transplant recipients for the first few weeks after transplant.
Methods:
Lethally irradiated B10.BR (H2k) mice were transplanted with 3 x 106 T cell depleted bone marrow cells (TCD-BM) alone or the combination of TCD-BM plus 2 x 106 purified T cells from B6 (H2b) donor mice. Mice received daily gavage with 100 mg/kg or 150 mg/kg ICA or vehicle through day 38 post-transplant. Survival and clinical manifestations of GvHD were monitored through day 62. Histopathology of the gut, cytokines in serum and colon homogenates, intracellular cytokine staining of donor T-cells and bacterial counts in mesenteric lymph nodes (using blood agar plates) were measured in a separate cohort of experimental mice euthanized on day 20. Additionally, a secondary transplant was conducted to test the allo-proliferative capacity (in vivo CFSE dilution assay) and GvHD activity of T-cells from spleens of mice in the TCD-BM + T cells + 150 mg/kg ICA group that survived to day 62, compared to B6 T cells, in combination with fresh B6 TCD-BM, in B10.BR recipients.
Results and Conclusion:
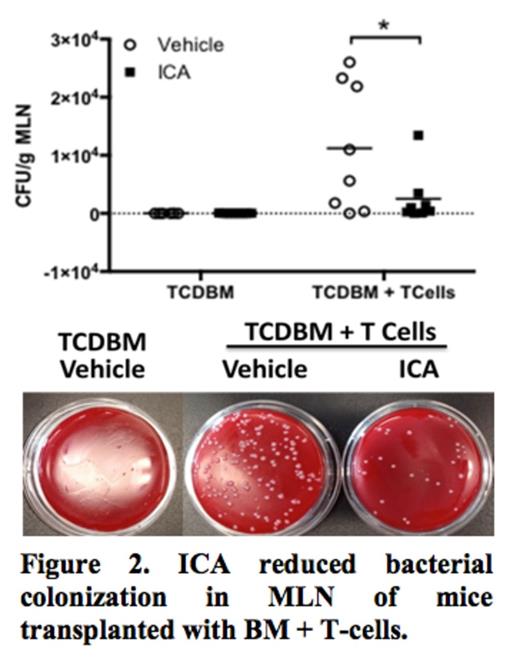
Figure 1 shows survival curves for mice transplanted with TCD-BM + T-cells that were fed vehicle (red), 100 mg/kg/day of ICA (blue), or 150 mg/kg/day of ICA (green) by oral gavage. Only 7% of the recipients of TCD-BM + T cells treated with vehicle only survived to day 46. ICA administration improved survival in a dose-dependent manner: recipients given 100 mg/kg/day of ICA had 20% survival at day 52 (**p = 0.009) and administration of 150 mg/kg/day of ICA resulted in 83% survival of recipients of TCD-BM + T cells (***p <0.001). ICA was well tolerated without serious toxicity in recipients of BM grafts without GvHD-causing T-cells (thin black solid line). ICA-treated recipients of TCD-BM + T cells had improved thymopoiesis and decreased levels of inflammatory cytokines. Histological analysis of colonic tissue from these ICA-treated recipients also showed marked reduction of GvHD pathology and decreased bacterial colonization of mesenteric lymph nodes (MLN) compared to vehicle-treated control animals (Figure 2, *p < 0.01), indicating enhanced gastrointestinal barrier function. T cells recovered from ICA-treated TCD-BM + T cell transplant recipients at day 62 had been rendered tolerant to recipient type alloantigens in secondary transplant in vivo assays as shown by reduced proliferation of CFSE-stained T cells and lack of GvHD activity in secondary B10.BR recipients.
These data indicate that enteral administration of ICA, a natural compound present in food, causes a dramatic reduction of GvHD in a MHC mismatched model of allogeneic bone marrow transplantation. We postulate that this effect is mediated through activation of the aryl hydrocarbon receptor in innate lymphoid cells in the lamina propria of the gut, stimulating regeneration of the gut epithelium and reducing trans-epithelial migration of gut bacteria that contribute to GvHD. Further development and exploration of the mechanism by which ICA treatment reduces GvHD could have a major impact on improving the safety of allogeneic bone marrow transplantation, and increase the availability of donors for those patients who currently lack an HLA matched sibling or volunteer donor.
No relevant conflicts of interest to declare.
Author notes
Asterisk with author names denotes non-ASH members.


This feature is available to Subscribers Only
Sign In or Create an Account Close Modal