Abstract
Background: Recent studies have demonstrated that AMG 330, a novel CD33/CD3-directed bispecific T-cell engaging (BiTE¨) antibody, is highly active against CD33+ AML cell lines and can potently lyse leukemic blasts from AML patients. Here, we have investigated the preclinical determinants for AMG 330 activity in primary newly diagnosed and relapsed/refractory human AML samples to prospectively understand factors that might contribute to clinical response or resistance.
Patients and Methods: Frozen aliquots of Ficoll-isolated mononuclear cells from peripheral blood or bone marrow specimens were obtained from adults with AML who consented to their use for research purposes. CD33 expression on myeloblasts (identified by appropriate CD45 and side-scatter properties) and the percentage of endogenous CD3+ T-cells were quantified by flow cytometry. To determine drug-induced cytotoxicity, AML cells were incubated in culture medium containing various concentrations of AMG 330; in some experiments, exogenous T-cells (isolated via magnetic cell sorting from a healthy adult volunteer who underwent leukapheresis) were labeled with CellVue Burgundy and added at different effector:target (E:T) cell ratios. After 48 hours, cell numbers and drug-induced cytotoxicity, using DAPI to detect non-viable cells, were determined by flow cytometry; AML cells were identified by forward/side scatter properties and negativity for CellVue Burgundy dye. Specific drug-induced cytotoxicity was calculated as: 100 x (1 – live target cellstreated/live target cellscontrol), and presented as mean±SEM.
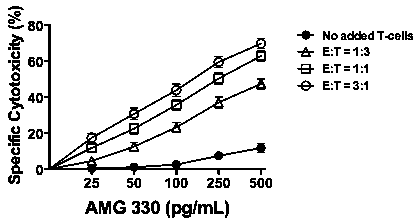
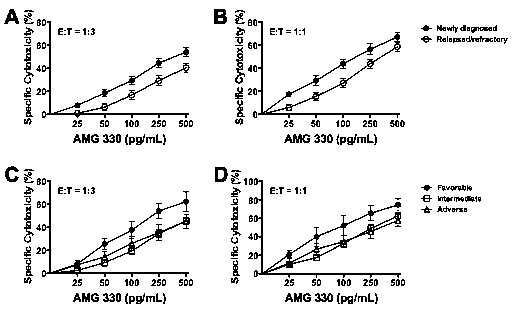
Results: Forty-one of the 49 studied specimens were included in our analyses as they had >40% myeloblasts upon thaw and were >50% and >30% viable at baseline and after 48 hours, respectively. Median age of the patients was 65.3 (range: 23.9-80.0) years. The median percentage of myeloid blasts and CD3+ T-cells in the studied specimens was 87.1% (range: 55.1-97.0%) and 2.0% (range: 0-27.3%), respectively, and median sample viability after 48 hours in culture was 76.7% (range: 31.1-93.5%). These characteristics were similar between the newly diagnosed (n=21) and relapsed/refractory (n=20) AML specimens. In the absence of healthy donor T-cells, AMG 330 resulted in modest cytotoxicity (at 500 pg/mL: 11.8±2.5%) that was correlated with the amount of autologous T-cells (at 500 pg/mL; r=0.566; p=0.0001; Spearman's rank correlation test). On the other hand, AMG 330 exerted marked cytotoxic activity in several specimens with very low CD33 expression, and there was no correlation between AMG 330 cytotoxicity and CD33 expression (at 500 pg/mL; r=-0.048; p=0.77). In the presence of healthy donor T-cells, AMG 330 cytotoxicity was strictly dependent on the drug dose (e.g. p<0.0001 at E:T=1:3) and E:T cell ratio (e.g. p<0.0001 at 500 pg/mL; Fig. 1). High cytotoxic activity of AMG 330 was observed in specimens with very low CD33 expression, and a correlation between AMG 330 cytotoxicity and CD33 expression was only observed at high E:T cell ratio (3:1) and higher AMG 330 doses (at 500 pg/mL: r=0.465, p=0.0022). In analyses of patient subsets, AMG 330 resulted in significantly higher cytotoxicity in specimens from patients with newly diagnosed AML (n=21) than those with relapsed/refractory disease (n=20) despite similar levels of CD33 on leukemic blasts (median of 837 [30-5,356] vs. 1,018 [7-2,567] arbitrary fluorescence units; Fig. 2A and B). Furthermore, AMG 330-induced cytotoxicity was higher in specimens from patients with cytogenetically/molecularly favorable-risk disease (n=5) as compared to those with intermediate- (n=26) or adverse risk disease (n=10; Fig. 2C and D).
Conclusion: AMG 330 causes potent cytolysis of primary human AML cells in vitro in a dose- and E:T cell ratio-dependent manner across the entire spectrum of cytogenetic/molecular risk and disease stage, even in specimens with very low expression of CD33. Lower activity of AMG 330 was observed in relapsed/refractory AML specimens (relative to newly diagnosed AML specimens) and intermediate- or adverse-risk disease specimens (relative to favorable-risk specimens) suggesting the potential presence of yet undefined, CD33-independent, relative resistance mechanisms in defined patient subsets.
Off Label Use: Use of AMG 330 in AML (AMG 330 is investigational drug). Newhall:Amgen, Inc: Employment. Sinclair:Amgen, Inc: Employment. Frankel:Amgen, Inc: Employment. Kischel:Amgen, Inc: Employment. Chen:Amgen, Inc: Employment. Walter:Amgen, Inc: Research Funding; Seattle Genetics, Inc.: Research Funding; Amphivena Therapeutics, Inc.: Consultancy; Covagen AG: Consultancy.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal