Abstract
Dipeptidylpeptidase 4 (DPP4) is a serine peptidase with enzymatic activity leading to the N terminal cleavage of select penultimate amino acids of proteins. We previously published that the number of cytokines, chemokines and growth factors that have putative DPP4 truncation sites have been dramatically underestimated. Functional and mechanistic roles of full length (FL) versus DPP4 truncated (T) factors, as well as the ability of DPP4 T proteins to induce signaling that FL factors can not, have not been previously investigated and may have yet unappreciated clinical application. Here we present novel data demonstrating the here to fore unknown ability of DPP4 cleavage of proteins to alter not only cellular function, but intracellular signaling of growth factors leading to miRNA expression, phosphorylation, and global induction of proteins that the FL form of the protein does not induce. Additionally, and unexpectedly, a 1:1 mixture of the DPP4 T protein and their FL counterpart leads to both overlapping signaling between the FL and T, as well as unique signaling, that is not induced by the FL or T protein alone. This suggests multifaceted, important, and currently unappreciated roles that the DPP4 truncation of proteins may play in the regulation of normal and malignant hematopoiesis as well as other physiologic and pathophysiologic states.
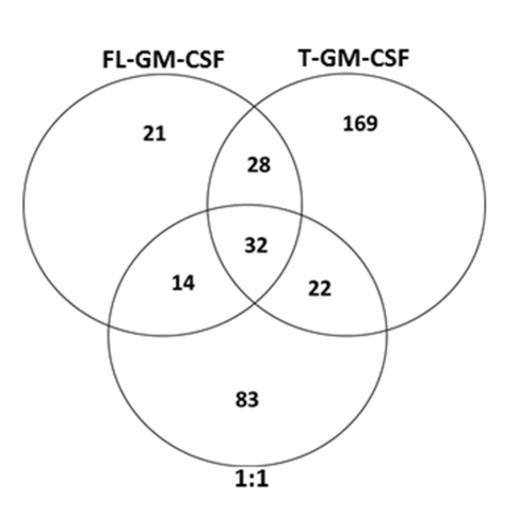
From functional data, utilizing the human factor-dependent TF-1 cell line, as well as primary samples from human cord blood (CB) and patients with Acute Myeloid Leukemia (AML), we observed that DPP4 truncation of GM-CSF (T-GM-CSF) and IL-3 (T-IL-3) results in decreased colony stimulating factor (CSF) activity in normal and malignant hematopoietic progenitor cells (HPC). Receptor binding studies confirmed that both T-GM-CSF and T-IL-3 have enhanced receptor affinity compared to their FL form and each can compete to blunt the receptor binding of either FL-GM-CSF or FL-IL-3. In vivo studies demonstrated that either exogenously added T-GM-CSF or T-IL-3 suppressed the effects of exogenously added FL-GM-CSF or FL-IL-3 on progenitor cell numbers per femur and diminished HPC cycling. Investigation of FL vs T mediated signaling alterations was done with TF-1 cells using proteomic and bioinformatic analysis of miRNA expression, and phosphorylation/global protein induction. Both T-GM-CSF and T-IL-3 induced unique signaling that FL-GM-CSF and FL-IL-3 did not, as well as signaling that overlapped with their FL counterparts (Figure 1, Venn Diagram example of global induction of proteins). Additionally, treatment with a 1:1 ratio of the FL/T proteins resulted in both distinctive, and common, signaling compared to that detected with treatment of only FL or T molecules, thus revealing the complexity of the signaling interactions and heretofore unknown activities of DPP4 truncated proteins (Figure 1). Fisher's exact test or B-H multiple testing correction in IPA software were used to statistically generate comparisons of molecules associated with specific signaling induction, and differential as well as overlapping signaling for all GM-CSF vs IL-3 groups. As examples, for both the GM-CSF and IL-3 groups, molecules associated with PI3K/Akt signaling were increased in T and 1:1 groups vs treatment with FL molecules. In contrast, molecules affiliated with CDC42 signaling were induced by treatment with a 1:1 ratio of FL:T molecules but not in the FL or T groups alone for both GM-CSF and IL-3. Interestingly, phosphorylated proteins associated with Flt3 and IL-9 signaling in hematopoietic progenitors was increased in the T and 1:1 groups for GM-CSF and IL-3 compared to the FL groups. Conversely, phosphorylated molecules involved in nucleic acid metabolism were induced by the FL, T and 1:1 of the GM-CSF groups but only by the FL version of IL-3. Further, phosphorylated molecules associated with antigen presentation were only detected in the FL IL-3 and 1:1 IL-3 groups, and molecules functionally associated with carbohydrate metabolism and cellular therapeutics were only detected in the T and 1:1 groups for both GM-CSF and IL-3, and not in the FL stimulated groups. Collectively, these data substantiate that further investigation into the roles and regulation of DPP4, in normal and malignant hematopoiesis, will allow for a better understanding of the significance, and potential clinical utility, of DPP4 activity altering compounds as well as DPP4 truncated molecules.
Broxmeyer:CordUse: Membership on an entity's Board of Directors or advisory committees.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal