Abstract
While allogeneic hematopoietic stem cell transplantation (alloHSCT) has extensively been studied in patients with AML <60 years of age, AML predominantly affects older individuals (median age at diagnosis of 71 years). The majority of older patients receiving intensive induction treatment may obtain a first complete remission (CR1), but the probability of relapse is very high necessitating effective post-remission treatment (PRT). AlloHSCT is the most effective PRT to prevent relapse, although non-relapse mortality (NRM) may compromise that beneficial effect, especially in the elderly. The development of reduced intensity conditioning (RIC) regimens, however, has enabled the application of alloHSCT in older patients with acceptable NRM. In the present study, we set out to compare alloHSCT following RIC with other PRTs including no further chemotherapy by time-dependent analysis in patients with AML aged 60 years and older.
A total number of 640 patients with newly diagnosed AML were included, with a median age of 66 years, who participated in consecutive, prospective HOVON-SAKK phase II/III trials (AML42/42A, AML43, AML81, and AML92) between May 2001 and February 2010 and who obtained CR1 after induction chemotherapy. PRT consisted of RIC alloHSCT (n=97), gemtuzumab ozogamicin (n=110), chemotherapy (n=38), autologous HSCT (autoHSCT) (n=23), or no further treatment (n=372). RIC regimens contained either busulfan and fludarabine, or 2.0 Gy total body irradiation preceded by fludarabine. Endpoints of this study were 5 year overall survival (OS), relapse-free survival (RFS), relapse and non-relapse mortality (NRM) measured from start of consolidation. Patients were classified by leukemia risk, based on the latest European Leukemia NET (ELN) risk classification. To perform a time-dependent statistical analysis of alloHSCT versus non-alloHSCT, a multivariable cox regression model with time-dependent covariates autoHSCT and alloHSCT was applied with adjustment for the different types of non-alloHSCT treatment, leukemia risk, white blood cell count at diagnosis, late CR, age, sex, and year of consolidation.
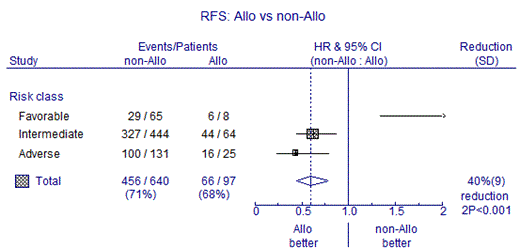
Recipients of alloHSCT were significantly younger as compared to patients who were not consolidated with alloHSCT (64 (range: 60-74) versus 67 (range: 60-82) years, respectively, p<0.001). Leukemia risk, white blood cell count at diagnosis, karyotyping, and CR reached after first or second cycle of chemotherapy, was not significantly different between the two groups. A trend towards improved OS was found for RIC alloHSCT recipients as compared to patients receiving no alloHSCT (35±5% versus 23±2% at 5 years, respectively, p=0.09), while OS was significantly improved in patients with adverse risk AML receiving an alloHSCT (34±10% versus 8±3% at 5 years, p=0.002). Recipients of RIC alloHSCT showed improved relapse-free survival (RFS), as compared to non-transplant approaches (32±5% versus 17±2% at 5 years, p=0.02), which effect was similarly exerted among intermediate and adverse risk AMLs. Following multivariable analysis with adjustment for covariates, RIC AlloHSCT was associated with better OS (HR 0.68, p=0.006), better RFS (HR 0.59, p<0.001), and less relapse (HR 0.44, p<0.001) as compared to non-transplant approaches (Figure 1). The cumulative incidence of NRM was 18±4% in the present study, demonstrating the feasibility of RIC alloHSCT in elderly patients. In the non-transplanted patients the estimated cumulative incidence of NRM was 10±1%.
Collectively, these results indicate that RIC alloHSCT significantly improves survival in elderly patients with AML in CR1 who are eligible for PRT upon achieving remission following induction chemotherapy. Subgroup analysis showed that, similar to results in younger patients, alloHSCT was not associated with better outcome in favorable risk patients, but both OS and RFS appeared improved in intermediate and poor risk patients. Our findings suggest that RIC alloHSCT is a feasible and effective PRT in elderly patients. Future studies including comorbidities and geriatric assessment may more reliably give insight into eligibility criteria other than age for alloHSCT in elderly patients. Our results underscore the importance of a prospective randomized study such as currently is being performed in Europe (http://clinicaltrials.gov/show/NCT00766779).
No relevant conflicts of interest to declare.
Author notes
Asterisk with author names denotes non-ASH members.


This feature is available to Subscribers Only
Sign In or Create an Account Close Modal