Abstract
Mutations in the DNA methyltransferase 3A (DNMT3A) gene are frequent in normal karyotype de novo acute myeloid leukemia (AML) (20-35%), chronic myelomonocytic leukemia (CMML) (10-20%) and myelodysplastic syndrome (MDS) (8%). Hematopoietic-specific loss of Dnmt3a in a mouse model leads to acquisition of aberrant self-renewal by the HSCs and expansion of the stem/progenitor compartment in bone marrow transplantation studies. Despite these important insights, the impact of hematopoietic deletion of Dnmt3a on disease phenotype in primary, non-transplanted mice has not been described.
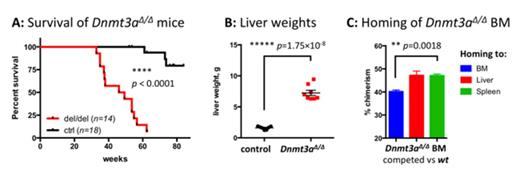
Mx1-Cre-mediated Dnmt3a ablation in the hematopoietic system in primary mice led to the development of a myeloproliferative neoplasm (MPN) with a 100% penetrance (n=14) and a median age of onset at 47.7 weeks (survival difference between Dnmt3a KO and control animals p<0.0001, Figure 1A). Loss of Dnmt3a in the hematopoietic compartment resulted in thrombocytopenia (platelet counts 250±251.8 K/μl in Dnmt3a KO vs 1260±292.8 K/μl in controls, p<0.002) and overall anemia (hematocrit 25.25±7.48% vs 44.8±5.83%, p<0.006). Marked expansion of the mature Mac1+Gr1+ myeloid cell population in the peripheral blood was evident by flow cytometric analysis (52.3±18.03% in Dnmt3a knock-outs).
Myeloproliferation induced by Dnmt3a loss was characterized by marked, progressive hepatomegaly (liver weights 7.25±1.195 g in Dnmt3a-deleted animals vs 1.61±0.266 g in wild-type controls, p<1.75×10^-8, Figure 1B) with moderate splenomegaly (spleen weights 457.5±379.6 mg vs 79.43±21.19 mg, p<0.033). Histopathological analysis revealed massive myeloid infiltration in spleens and livers leading to complete effacement of organ architecture, left shifted myeloid cells, and occasional blasts. In addition, the presence of megakaryocytes in spleens and livers of Dnmt3a-deleted mice was indicative of extramedullary hematopoiesis. The significant myeloid infiltration of liver parenchyma was confirmed by flow cytometric analysis of liver tissue, with Mac1+Gr1+ myeloid cells making up 66.15±11.93% of all viable cells. In line with previous reports, we observed an increased number of immunophenotypically defined stem (Lin-Sca1+cKit+, LSK, 2.013±1.200% in Dnmt3a-ablated mice vs 0.423±0.052% in controls, a 4.76-fold increase, p<0.014) and granulomonocytic progenitor (GMP, Lin-Sca1-cKit+CD34+FcγR+, 2.713±1.593% vs 1.278±0.451%, a 2.12-fold increase, p<0.024) cells in the bone marrow. Consistent with extramedullary hematopoiesis, we were able to detect expanded LSK cell populations in livers and spleens of Dnmt3a-deleted mice.
Notably, the myeloid disease phenotype induced by Dnmt3a loss was fully transplantable, including the marked hepatomegaly; these data demonstrate that the liver-specific expansion reflects a cell-autonomous mechanism. To assess relative tropism for different target organs, we next performed homing studies where Dnmt3a-deleted bone marrow cells were competed against wild-type counterparts in lethally irradiated hosts. 48 hours after transplantation, we observed increased tropism of the Dnmt3aΔ/Δ BM cells to the liver and spleen, whereas control cells preferentially localized to the bone marrow (difference between homing to bone marrow and spleen/liver p<0.0115, Figure 1C). These data demonstrate that altered homing and tissue tropism of Dnmt3a KO hematopoietic cells promote extramedullary hematopoiesis and liver involvement. ERRBS and gene expression profiles by RNA-seq in stem and progenitor cell populations demonstrated differential regulation of key biologic pathways, including self-renewal, hematopoietic lineage commitment and differentiation, and heterotypic cell-cell interactions.
In conclusion, our studies show that ablation of Dnmt3a in the hematopoietic system leads to myeloid transformation in vivo, with cell autonomous liver tropism and marked extramedullary hematopoiesis. These data demonstrate, in addition to its established role in controlling self-renewal, Dnmt3a serves as an important regulator of the myeloid compartment that limits expansion of myeloid progenitors in vivo.
No relevant conflicts of interest to declare.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal