Abstract
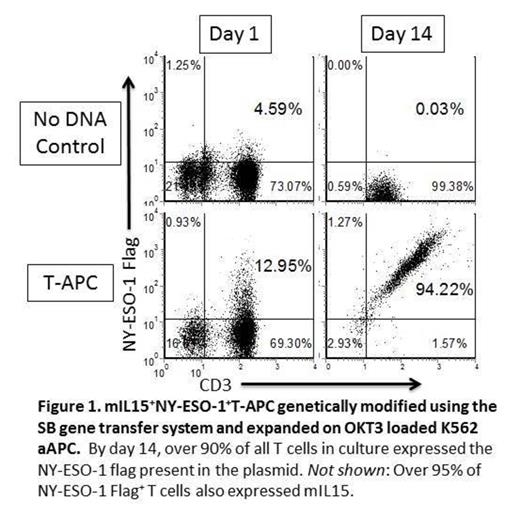
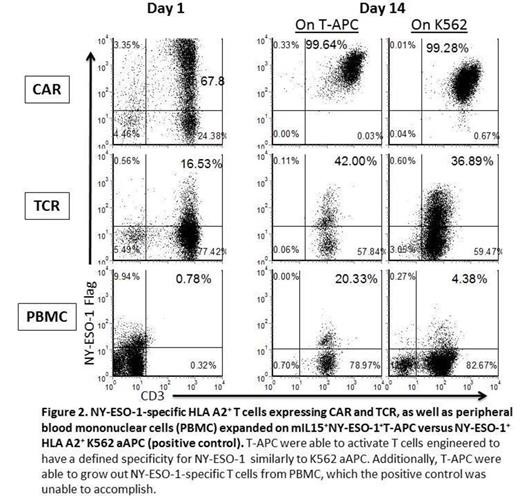
T cells have been previously genetically manipulated to act as a cellular vaccine to present viral antigens such as MP1 from influenza A. We have now genetically modified T cells as antigen presenting cells (T-APC) to express the tumor-associated antigen (TAA) NY-ESO-1 (Figure 1 ). This TAA is reportedly found in the majority of patients with high risk multiple myeloma, as well as other malignancies, but expression is absent from normal tissues. Therefore, immunologic targeting of NY-ESO-1 may be a potential treatment strategy for myeloma that is resistant to conventional therapies. One approach to improve therapeutic outcome is by adoptive transfer of T cells rendered specific for a TAA preferentially expressed on tumor cells. T-cell specificity can be redirected to intracellular TAAs by enforced expression of a characterized T-cell receptor (TCR) or chimeric antigen receptor (CAR) that recognizes processed antigen in the context of restricting human leukocyte antigen (HLA). However, persistence of these infused genetically modified cells, which is directly related to a favorable clinical response, may be variable. Therefore, T-APC may have a dual function of increasing persistence of adoptively transferred CAR+ and/or TCR+ T cells as well as inducing long term immunity through direct and cross priming of endogenous immune cells. To enhance these presentation functions, a new co-stimulatory molecule that tethers IL-15 to the cell surface (membrane-bound IL-15, mIL15) was expressed on NY-ESO-1+T-APC using the Sleeping Beauty (SB) gene transfer system. NY-ESO-1-specific HLA A2+ T cells expressing TCR and CAR could be selectively propagated ex vivo on autologous mIL15+NY-ESO-1+T-APC. The T-APC were also able to selectively propagate NY-ESO-1-specific T cells from autologous peripheral blood which can be described by binding of specific pentamer (Figure 2 ). Our data demonstrate that it is feasible to generate T-APC that can activate T cells engineered to have a defined specificity for NY-ESO-1 as well as grow out NY-ESO-1-specific T cells. The SB system is in place for genetic modification of clinical-grade T cells to express CAR, TCR, NY-ESO-1, and mIL15. Thus, we are proceeding to a human trial to infuse T-APC with and without genetically modified T cells in patients with NY-ESO-1+ tumors such as myeloma.
Cooper:InCellerate: Equity Ownership; Sangamo: Patents & Royalties; Targazyme: Consultancy; GE Healthcare: Consultancy; Ferring Pharmaceuticals: Consultancy; Fate Therapeutics: Consultancy; Janssen Pharma: Consultancy; BMS: Consultancy; Miltenyi: Honoraria.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal