Abstract
Myeloproliferative disorders (MPDs) are heterogeneous clonal diseases with variable clinical courses. Mutations in the Cbl family genes have been reported in multiple independent studies to be present in about 10% of patients with MPDs and these patients tend of have a poorer prognosis. We have previously demonstrated that Cbl-flox/flox, Cbl-b-null mice rendered Cbl/Cbl-b double knockout (DKO) in the hematopoietic compartment with MMTV-Cre develop a disease phenotypically similar to human MPDs. An in vitro kinase inhibitor screen identified fasudil, a ROCK inhibitor, with relatively selective anti-proliferative activity against Cbl/Cbl-b DKO vs. control murine bone marrow cells. We established a mouse model with a uniform time of MPD onset by transplanting Cbl/Cbl-b DKO bone marrow cells (2x106 per recipient) into 21 busulfan-conditioned NOD/SCID/gamma chain-deficient (NSG) mice. All recipients showed donor cell engraftment. Four weeks post-transplant, we treated 13 mice with 100 mg/kg fasudil daily by gavage. By two weeks of treatment, total white cell and monocyte counts were significantly lower in mice treated with fasudil (p=0.02 and 0.04 respectively). For the entire group, we observed a trend towards improved survival in fasudil-treated mice that didn't reach statistical significance (p=0.07). However, analysis of male recipients only (n=12) revealed a significant survival advantage in fasudil-treated group (p=0.04). The gender difference may stem from a currently unexplained more severe disease phenotype we observed in female recipients. Notably, while all untreated mice succumbed to MPD, prolonged survival was observed in 2 mice beyond 27 weeks, nearly twice the average life-span in the Cbl/Cbl-b DKO MPD model (16 weeks). The 2 long-term survivors had undetectable levels of myosin light chain (MLC), a downstream target of ROCK phosphorylation (figure) suggesting that inhibition of downstream signaling of ROCK is associated with improved disease control and survival. Taken together, our data suggest a therapeutic potential for fasudil in the treatment of MPDs or other mutant Cbl-driven myeloid disorders.
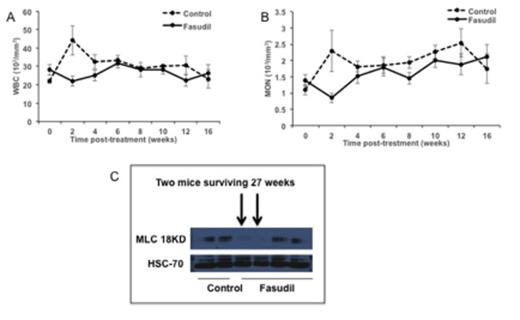
Fasudil activity in mouse model of Cbl/Cbl-b DKO MPD. Total WBC (A) and monocyte (B) counts over time in untreated and fasudil-treated mice (mean+/-SEM), C: MLC levels by Western Blot in peripheral blood of control vs. fasudil-treated mice; those surviving longer than 27 weeks are indicated.
Fasudil activity in mouse model of Cbl/Cbl-b DKO MPD. Total WBC (A) and monocyte (B) counts over time in untreated and fasudil-treated mice (mean+/-SEM), C: MLC levels by Western Blot in peripheral blood of control vs. fasudil-treated mice; those surviving longer than 27 weeks are indicated.
No relevant conflicts of interest to declare.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal