Abstract
Background: Infusion related reactions (IRR) are commonly seen with administration of biologic therapies but remain relatively poorly understood. Rituximab is thought to induce cell death upon binding to CD20 primarily by complement dependent cytotoxicity (CDC) and antibody-dependent cell-mediated cytotoxicity (ADCC). Obinutuzumab (GA101, Gazyva®, Gazyvaro®) is a new generation humanised IgG1 Type II anti-CD20 mAb with contrasting properties to rituximab. The Fc portion has been glycoengineered (afucosylated) to increase binding affinity to Fcgamma receptor 3(FcγRIII). These features result in higher direct cell killing activity, enhanced ADCC and phagocytosis (ADP) and less complement activation compared with rituximab.
Early experience with rituximab has shown that release of pro-inflammatory cytokines occurs with initial administration and is higher in patients experiencing IRRs compared to those who are not. We report on the cytokine release in a subset of 38 patients with chronic lymphocytic leukemia (CLL) treated with obinutuzumab monotherapy pooled from the earlier Phase I (GAUSS) trial (NCT00576758) and Phase I/II (GAUGUIN) trial (NCT00517530).
Methods: Eligible patients for both studies had relapsed/refractory CLL with no alternative, higher priority therapy available. All patients were treated sequentially with intravenous (IV) obinutuzumab monotherapy and were universally pre-medicated with an oral anti-pyretic (paracetamol/acetaminophen) and an IV anti-histamine 30 minutes prior to starting the infusion. Steroid pre-medication was not mandated in either study.
Cytokine serum samples (quantitatively measured using the Becton-Dickinson (BD) Cytometric Bead Array) were taken from each patient prior to each obinutuzumab infusion, mid-way through the infusion, at the end of infusion and 2-5 hours after completion. Samples for complement analysis were also taken at these same time points (C3/C4 analysed using Siemens BNII Nephelometer, C3a/C5a using BD Pharmingen ELISA kit). Peripheral blood samples for immunophenotyping were also taken at baseline, prior to and at the end of each infusion. The development of an IRR with the first infusion was defined as the occurrence of related signs and symptoms within 24 hours of administration of antibody and graded according to the CTCAE guidelines v4.0.
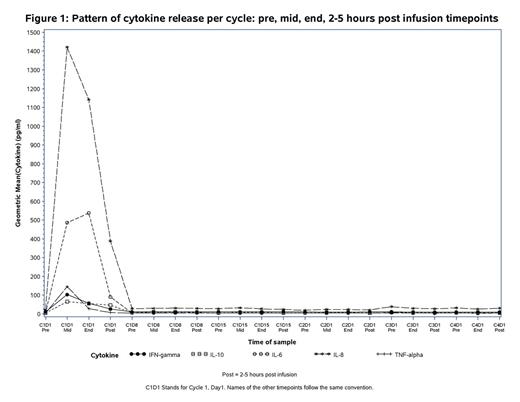
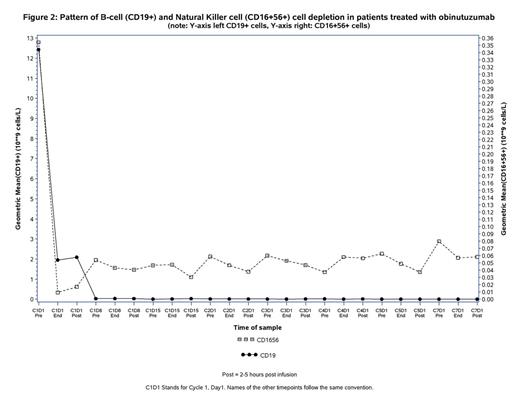
Results: Of the 38 patients treated, 35 developed symptoms of IRR (Grade 1/2=25, Grade 3/4=10) with the first infusion accompanied by a rapid decrease in circulating CD19+ B cells, a drop in the measurable natural killer (NK) CD16+56+ cells and an increase of pro-inflammatory cytokines IL6, IL8, TNFα and IFNγ (see figures 1 and 2). There were no meaningful differences between pre-treatment baseline cytokine levels across all subgroups. At the mid-infusion time-point, patients with absolute lymphocyte count (ALC) at baseline ≥50x109/L released higher levels of IL6 (mean IL6(log10) 3.16 vs 2.41), IL-8 (mean IL8(log10)3.57 vs 2.91), TNFα (mean TNFα(log10) 2.59 vs 1.92), IFNγ (mean IFNγ(log10)2.38 vs 1.8) and IL10 (mean IL10(log10) 2.03 vs 1.69) than those with ALC <50x109. Patients who had higher grade IRR (≥grade 3) had higher baseline values for lymphocyte count and β-2microglobulin, lower baseline platelet count and higher Binet stage than those without severe grade IRR, however none of these differences were statistically significant at p≤0.05. . Markers of complement activation, C5a and C3a, did not increase and C3/C4 levels remained stable across all groups. Cytokine release was limited to the first infusion of obinutuzumab only and did not recur with subsequent infusions (Fig . 1).
Conclusions: This sub-analysis demonstrates that the first obinutuzumab administration triggers immediate and strong release of cytokines (IL6, IL8, TNFα, IFNγ and IL10) in association with rapid destruction of circulating B cells in patients with CLL. The close temporal relationship between release of pro-inflammatory cytokines (especially IL6 and IL8) and development of IRRs, in addition to the lack of complement activation induced by obinutuzumab, suggest that cytokine release is part of the IRR pathophysiology. Intervention strategies targeting cytokines may therefore be a promising strategy to reduce the incidence and severity of IRRs.
Freeman:Roche Pharmaceuticals: clinical research fellowship supported by Roche Pharmaceuticals (secondment from Bart's) Other. Off Label Use: Presentation may refer to mitigation strategies such as the use of tocilizumab in patients with cytokine release syndrome. Morschhauser:Bayer: Honoraria; Mundipharma: Honoraria; Genentech: Honoraria; Spectrum: Honoraria; Gilead: Honoraria. Sehn:Roche: Research Funding. Dixon:Roche Pharmaceuticals: Employment. Houghton:Roche Pharmaceuticals: Employment. Fingerle-Rowson:Roche Pharmaceuticals: Employment. Wassner-Fritsch:Roche Pharmaceuticals: Employment. Hallek:Roche Pharmaceuticals: Consultancy, Research Funding, Speakers Bureau. Salles:Roche Pharmaceuticals: Honoraria, Research Funding. Cartron:Roche: Consultancy, Honoraria.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal