Abstract
Introduction
Combination of total body irradiation, fludarabine, cytarabine and idarubicine (TBI/Flu/AraC/Ida) is a disease-specific myeloablative conditioning regimen used in patients with acute myeloid leukemia. We report a single centre retrospective analysis of outcomes of allogeneic hematopoietic cell transplantation (allo-HCT) in patients with AML treated with TBI/Flu/AraC/Ida in comparison to TBI/Cy.
Methods
Patients in the TBI/Flu/AraC/Ida arm received 12 Gy total body irradiation in 6 fractions on days -3 to -1, fludarabine 25mg/m2 on days -7 to -1, cytarabine 2000mg/m2 on days -7 to -3 and idarubicine 10mg/m2 on days -7,-5 and -3. Patients in TBI/Cy arm received 12Gy TBI in 6 fractions on days -3 to -1 and cyclophosphamide 60mg/kg on days -7 and -6.
GvHD prophylaxis consisted of cyclosporine and methothrexate prior to year 2004. Since 2004, GvHD prophylaxis included cyclosporine with mycophenolate mofetil and ATG-Fressenius 20 – 40mg/kg was given to patients with unrelated donors.
Results
Sixty four patients with AML received allo-HCT using TBI/Flu/Ida/AraC conditioning regimen between 2004 and 2012. Fifty three patients received TBI/Cy conditioning between 1987 and 1998 and between 2009 and 2014. Median age was 46 (range: 19-60) and 42 (range: 14-59) TBI/Flu/Ida/AraC and TBI/Cy, respectively.
In the TBI/Flu/Ida/AraC group, 19 (30%) of patients received transplant from a HLA matched sibling donor, 16 (25%) from a HLA matched unrelated donor and 29 (45%) from HLA mismatched unrelated donor. In the TBI/Cy group, 24 (45%) of patients received transplant from a HLA matched sibling donor, 20 (38%) from a HLA matched unrelated donor and 9 (17%) from HLA mismatched unrelated donor (p = 0.0049). Other baseline patient and donor characteristics (patient/donor gender, CMV serology, AB0 matching, cytogenetic risk group, disease status and HCT-CI comorbidity score, ATG use) were not significantly different between the groups.
Median time to neutrophil engraftment was 18 days (range 10 - 31) and 21 days (range 12 – 32) in TBI/Flu/AraC/Ida a TBI/Cy groups, respectively. Median time to platelet engraftment to more then 20G/l was 21 (range 12 – 103) and 22.5 (range 9 – 54) days, respectively. Among patients who survived more than 100 days, 2/45 patients did not engraft platelets in the TBI/Flu/AraC/Ida group and 3/35 in the TBI/Cy group.
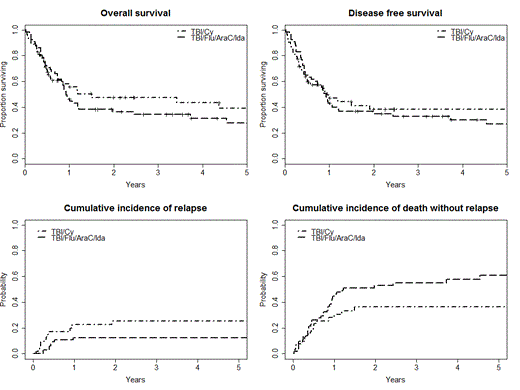
Overall survival (OS) at 2 years was 35% (95% CI: 25 – 49%) in TBI/Flu/Ida/AraC group and 47.5% (95% CI: 35 – 65%) in TBI/Cy group. Two years disease free survival (DFS) was 35% (95% CI: 25 - 49) in TBI/Flu/Ida/AraC group and 40% (95% CI: 27 - 58) in TBI/Cy group.
Cumulative incidence of relapse at 2 years after transplant was 12.5% and 25% (p = 0,08), and the cumulative incidence of non-relapse mortality was 53% and 36% (p = 0.059) in the TBI/Flu/Ida/AraC and TBI/Cy groups, respectively.
Survival curves and relapse incidence curves are presented in Figure 1.
In multivariate analysis of the influence of baseline characteristics and conditioning regimen on OS and DFS, only HLA mismatch was significantly associated with increased risk of mortality with HR 2,22 (95% CI: 1,25 – 3,95). HR of death was 1.11 (95% CI: 0.62 – 1.98) and HR of death or relapse was 0.87 (95% CI: 0.50 - 1.52) in patients who recieved TBI/Flu/Ida/AraC.
There was a trend towards increased incidence of acute graft versus host disease (aGVHD) grade II-IV in the TBI/Flu/AraC/Ida group (p = 0,09). The cumulative incidence of aGVHD was 46% and 34% in the TBI/Flu/AraC/Ida and TBI/Cy groups, respectively. Estimates of the cumulative incidence of aGVHD in the presence of competing risk of relapse or death are shown in Figure 2.
Conclusion
TBI/Flu/Ida/AraC regimen led to similar OS and DFS as TBI/Cy. There was a trend towards decreased rate of relapse, but increased non-relapse mortality with the TBI/Flu/Ida/AraC conditioning. In multivariate analysis, only HLA mismatch had significant effect on patient outcome.
The retrospective nature of the study and inequalities in baseline transplant characteristics, mainly more frequent use of mismatched unrelated donors in the TBI/Flu/Ida/AraC group, are main limitations of the study.
Off Label Use: Fludarabine, Cytarabine and Idarubicine as part of conditioning regimen.
Author notes
Asterisk with author names denotes non-ASH members.
This icon denotes a clinically relevant abstract

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal