Abstract
Graft versus host disease (GVHD) remains a major limitation of allogeneic hematopoietic stem cell transplantation (allo-HSCT), and gut GVHD specifically is a major cause of GVHD-related morbidity and mortality. Little is known about regulation of the intestinal stem cell (ISC) compartment in gut GVHD. We have found that Interleukin-22 (IL-22) produced by innate lymphoid cells is important for ISC recovery after transplant. However, the mechanism of action and specific cellular targets of IL-22 leading to ISC recovery are poorly understood.
Using clinically modeled LP into C57BL/6 (B6) minor antigen-mismatched HSCT (H-2 into H-2b), we found that daily treatment with recombinant murine (rm)IL-22 (4ug, intraperitoneal injection) starting day seven after transplant led to reduced intestinal pathology from GVHD without altering alloreactive immunity. Both overall GVHD pathology and epithelial apoptosis scores were significantly lower three weeks post-BMT in rmIL-22-treated mice with GVHD compared to PBS-treated controls (p<0.001). We observed that mice treated with rmIL-22 (and no pharmacologic immunosuppression) had increased numbers of Lgr5+ ISCs and significantly greater ISC proliferation (p<0.01). This was not due to IL-22-dependent changes in the ISC niche, as Paneth cell numbers, Paneth cell-derived growth factors (EGF, Wnt3), and stroma-derived growth factors (Rspo3) were all unchanged after IL-22 administration. However, the antimicrobial proteins Reg3β and Reg3γ were both upregulated by qPCR in small intestine (SI) of rmIL-22-treated mice (p<0.01 and p<0.001 respectively), although this did not result in consistent changes in the gut microbial flora.
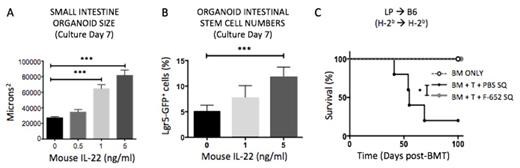
To evaluate direct effects on epithelial regeneration, we performed intestinal organoid culture assays in the presence of IL-22. Organoids generated from SI and large intestine (LI) crypts of wild-type B6 mice demonstrated substantially increased size after seven days of culture with IL-22 (p<0.001, SI, Fig. 1A; p<0.05, LI). Co-culturing crypts with innate lymphoid cells (ILC), potent producers of IL-22 in vivo, led to increased organoid size as well. Furthermore, culture with IL-22 significantly increased organoid budding (new crypt formation), resulting in increased organoid expansion with serial passaging in the presence of IL-22 (1ng/ml) suggesting that IL-22 could directly increase ISC expansion. Indeed, IL-22 culture led to increased organoid EDU incorporation and expansion of Lgr5+ ISCs after culture of SI crypts from Lgr5-GFP reporter mice (p< 0.001, Fig. 1D). Demonstrating a direct effect on ISCs, IL-22 led to STAT3 phosphorylation specifically in Lgr5+ cells and resulted in increased budding of organoids cultured from isolated single SI ISCs after only four days in culture (p<0.01).
To investigate the translational potential for use in humans, we tested a human IL-22 dimer/Fc fusion molecule (F-652, Generon Corp., Shanghai) on mouse intestinal crypts and found that F-652 significantly increased the size of SI and LI organoids. Using the LP into B6 allo-HSCT model described above, we found that every other day subcutaneous (SQ) treatment with 100 ug/kg F-652 starting day seven post-BMT led to significant improvement in both clinical GVHD score (P<0.0001) and survival (p<0.05, Fig. 1C).
In summary, we found that IL-22 and innate lymphoid cells can bridge immune function and tissue regeneration by acting directly on epithelial stem cells. IL-22 and F-652 therapy may represent a novel approach to promote intestinal recovery in patients with GVHD without increasing post-transplant immunodeficiency.
No relevant conflicts of interest to declare.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal