Abstract
Umbilical cord blood (UCB) is an alternative source of hematopoietic stem cells for patients without HLA-matched adult donors. UCB contains a low number of nucleated cells and mostly naive T cells, resulting in prolonged time to engraftment and lack of transferred T-cell memory. Although the first phase of T-cell reconstitution after UCB transplantation (UCBT) depends on peripheral expansion of transferred T cells, permanent T-cell reconstitution is mediated via a central mechanism, which depends on de novo production of naive T lymphocytes by the recipient’s thymus from donor-derived lymphoid-myeloid progenitors (LMPs). Thymopoiesis can be assessed by quantification of recent thymic emigrants, T-cell receptor excision circle levels, and T-cell receptor repertoire diversity. These assays are valuable tools for monitoring posttransplantation thymic recovery, but more importantly they have shown the significant prognostic value of thymic reconstitution for clinical outcomes after UCBT, including opportunistic infections, disease relapse, and overall survival. Strategies to improve thymic entry and differentiation of LMPs and to accelerate recovery of the thymic stromal microenvironment may improve thymic lymphopoiesis. Here, we discuss the mechanisms and clinical implications of thymic recovery and new approaches to improve reconstitution of the T-cell repertoire after UCBT.
The first successful umbilical cord blood (UCB) transplantation (UCBT) was performed in 1988, using cord blood from an HLA-matched sibling in a patient with Fanconi anemia.1 Since then, multiple large-scale trials have proven the clinical utility of UCBT as an alternative source of hematopoietic stem cells (HSCs) for the treatment of children and adults who are in need for allogeneic HSC transplantation (HSCT).2-4 Compared with peripherally mobilized HSC or bone marrow (BM) HSC units from unrelated adult donors, UCB grafts have the advantage of immediate availability, absence of risk to the donors, and lower immunogenicity, which allows for a greater degree of HLA incompatibility.5 UCB contains mostly naive, antigen-inexperienced T lymphocytes which do not transfer protective T-cell memory function to the host. Additionally, UCB T cells display impaired capacity for effector cytokine production and reduced cytolytic activity.6,7 Furthermore, UCB contains high numbers of T regulatory cells (Tregs) with a more potent suppressor function compared with adult Tregs.8 Consequently, UCBT is associated with delayed and incomplete immune reconstitution due to the lack of transferred adoptive immunity and due to the delayed HSC engraftment and reconstitution of lymphopoiesis in the host thymus.9 As a result, infectious complications and viral reactivation remain the most important causes of peritransplant morbidity and mortality in UCBT recipients.
Mechanisms of T-cell reconstitution after allogeneic HSCT
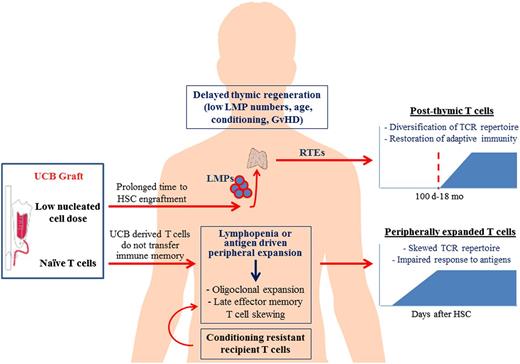
Allogeneic transplantation is followed by a period of profound lymphopenia and immunodeficiency, as a result of conditioning chemotherapy and use of immunosuppressive agents to prevent graft rejection or GVHD. The quantitative and qualitative reconstitution of the T-cell compartment is a slow process that can extend beyond the first year after HSCT and proceeds along 2 different pathways that act in parallel but follow distinct kinetics10 (Figure 1). In the early posttransplant period, the thymus-independent pathway predominates and is mediated by adoptively transferred donor T cells contained in the graft or recipient T cells that survive conditioning. These transferred T lymphocytes undergo homeostatic expansion in response to lymphopenia and high cytokine levels, which characterize the early posttransplant period, or oligoclonal proliferation upon interaction with cognate antigens. In contrast to peripherally mobilized HSC or BM grafts from adult donors, which are characterized by oligoclonal T-cell receptor (TCR) profiles and contain a considerable number of memory T cells, UCB grafts contain uniformly antigen-inexperienced T cells, which display a complete TCR repertoire at birth.11 The lymphopenia-driven homeostatic expansion of UCB T cells after transplantation leads to gradual loss of the naive phenotype and transition to an effector or memory-like phenotype.12,13 In addition, the antigen-driven peripheral T-cell expansion results in the contraction of T-cell repertoire diversity. These changes in the composition of the T-cell pool early after UCBT lead to an increasingly contracted and skewed T-cell repertoire that cannot sustain immune protection against a broad spectrum of antigens.
Reconstitution of the T-cell compartment after UCBT. Conditioning chemoradiation prior to UCBT results in profound lymphopenia and immunodeficiency of the host. T-cell reconstitution after UCBT is achieved by 2 independent mechanisms: the thymus-independent pathway of T-cell reconstitution predominates in the early posttransplant period and is mediated by adoptively transferred UCB T cells, which are uniformly naive and do not transfer protective immune memory, or recipient T cells that survive conditioning. These T-cell populations undergo peripheral expansion in response to lymphopenia and high cytokine levels (IL-7, IL-15, etc), or oligoclonal proliferation upon interaction with cognate antigen. Overtime, this early peripheral T-cell expansion results in late effector memory T-cell skewing and contraction of the T-cell repertoire diversity, and is associated with impaired immunologic responses to antigens. Reconstitution of a functionally competent T-cell compartment with broad antigenic specificity eventually requires the de novo production of naive T cells by the thymus of the UCBT recipient. This thymus-dependent pathway of T-cell reconstitution is a prolonged multistep process. LMPs contained in the UCB graft or arising from the engrafted donor-derived HSCs migrate via circulation and repopulate the thymus with thymocyte precursors that can reconstitute thymopoiesis. The thymus provides the essential microenvironment (stroma) that supports T-cell proliferation, selection, and differentiation into RTEs. Several factors can delay the recovery of thymopoiesis after UCBT, including low number of LMPs (as a result of low nucleated cell dose of UCB and delayed engraftment), advanced recipient age with resultant thymic involution and thymic damage from the conditioning chemoradiation or GVHD. Although slow, the thymus-dependent mechanism is imperative for the renewal of peripheral T-cell pool and constant export of new naive T cells with broad TCR repertoire diversity, capable of responding to a great spectrum of antigens.
Reconstitution of the T-cell compartment after UCBT. Conditioning chemoradiation prior to UCBT results in profound lymphopenia and immunodeficiency of the host. T-cell reconstitution after UCBT is achieved by 2 independent mechanisms: the thymus-independent pathway of T-cell reconstitution predominates in the early posttransplant period and is mediated by adoptively transferred UCB T cells, which are uniformly naive and do not transfer protective immune memory, or recipient T cells that survive conditioning. These T-cell populations undergo peripheral expansion in response to lymphopenia and high cytokine levels (IL-7, IL-15, etc), or oligoclonal proliferation upon interaction with cognate antigen. Overtime, this early peripheral T-cell expansion results in late effector memory T-cell skewing and contraction of the T-cell repertoire diversity, and is associated with impaired immunologic responses to antigens. Reconstitution of a functionally competent T-cell compartment with broad antigenic specificity eventually requires the de novo production of naive T cells by the thymus of the UCBT recipient. This thymus-dependent pathway of T-cell reconstitution is a prolonged multistep process. LMPs contained in the UCB graft or arising from the engrafted donor-derived HSCs migrate via circulation and repopulate the thymus with thymocyte precursors that can reconstitute thymopoiesis. The thymus provides the essential microenvironment (stroma) that supports T-cell proliferation, selection, and differentiation into RTEs. Several factors can delay the recovery of thymopoiesis after UCBT, including low number of LMPs (as a result of low nucleated cell dose of UCB and delayed engraftment), advanced recipient age with resultant thymic involution and thymic damage from the conditioning chemoradiation or GVHD. Although slow, the thymus-dependent mechanism is imperative for the renewal of peripheral T-cell pool and constant export of new naive T cells with broad TCR repertoire diversity, capable of responding to a great spectrum of antigens.
Reconstitution of a functionally competent T-cell compartment with broad antigenic specificity depends on the de novo production of naive T cells by the thymus of the HSCT recipient.14 This thymus-dependent pathway is a prolonged process, which begins with the migration of early lymphoid-myeloid progenitors (LMPs), present in the graft or arising from donor-derived HSCs after engraftment, which circulate in the periphery and seed the recipient’s thymus. LMPs initially settle in the cortex where they undergo expansion and T-cell lineage commitment under the influence of Notch signaling.15 Furthermore, the thymus provides the essential microenvironment (stroma) that supports T-cell proliferation, differentiation, and selection via cytokines (interleukin-7 [IL-7], stem cell factor [SCF], keratinocyte growth factor [KGF], C-C motif chemokine ligand 25 [CCL25]) and surface proteins (chemokine receptors, peptide/major histocompatibility complex [MHC] complexes), which facilitate trafficking and cell-cell interactions.16 Thymocytes undergo sequential rearrangements of the loci encoding for the β- and α-chains of TCR, which result in the assembly of the αβ-TCR and are subject to positive selection. Positively selected thymocytes undergo differentiation into CD4 and CD8 single-positive (SP) thymocytes, according to the restriction of their TCR to recognize either MHC class II or I molecules, respectively. Finally, SP cells migrate to the medulla and undergo negative selection, a process that assures the elimination of self-reactive thymocytes. Only the small percentage of thymocytes that have successfully undergone positive and negative selection exit the thymus and are termed recent thymic emigrants (RTEs).
Regardless of the graft source, the efficiency of thymopoiesis after HSCT depends on several factors, including recipient age, intensity of the conditioning regimen, and graft-versus-host disease (GVHD). In UCBT, 2 additional factors are of particular importance. First, the low number of hematopoietic progenitor cells in the UCB graft results in delayed engraftment and emergence of LMPs that migrate to the thymus and reinitiate thymopoiesis. Second, the greater degree of MHC discordance between donor and host in UCBT compared with HSCT from adult donors, might adversely affect intrathymic T-cell selection, which occurs in an MHC-restricted manner. Specifically, during positive selection, cortical thymic epithelial cells (cTECs) expressing self-MHC mediate presentation of self-peptides and select TCRs with intermediate affinity for such antigens. Subsequently, T cells surviving positive selection are challenged during negative selection in the thymic medulla for reactivity with self-peptides presented by medullary epithelial cells (mTECs) or by BM-derived dendritic cells (DCs). Although after allogeneic HSCT, cTECs and mTECs remain of host origin, thymic DCs are of donor origin. Consequently, it has been proposed that the degree of MHC incompatibility between donor and host can adversely impact thymic-dependent T-cell reconstitution, resulting in altered peripheral T-cell repertoire due to perturbation of the physiologic mechanisms of the positive selection process in the thymus.17 This hypothesis has been supported by clinical findings showing that TCR repertoire diversity is only mildly skewed in recipients of BM from HLA-identical related donors, whereas recipients of BM from HLA-mismatched related donors or matched unrelated donors (MUDs) display a markedly skewed TCR repertoire during the first year after transplantation.14,18,19 In that regard, because the lower alloreactivity of UCB grafts allows for a greater degree of MHC discordance between donor and host, the markedly skewed TCR repertoire diversity in UCBT recipients during the first year after UCBT, might be a consequence of a perturbed thymic selection process due to the greater degree of MHC discordance between UCB and host.
Quantitative assessment of thymopoiesis in humans
Maintenance of a broad TCR repertoire depends on the constant export of naive T cells from the thymus, which persists throughout adult life.20 In the past, radiographic imaging had been used to monitor the size of the thymus as a surrogate marker of thymopoiesis. However, size alone does not reliably reflect the actual functional capacity of the organ. Consequently, volumetric studies have considerable limitations in the evaluation of thymic recovery. Identification of thymus-derived naive T cells by immunophenotyping provides a more accurate assessment of thymic activity and RTE export. The expression of CD45 isotopes has been most widely used for the enumeration of the naive (CD45RA+) T cells, although not always reliably.21 The use of additional surface markers, including CCR7, CD62L, CD31, CD27, and αEβ7 integrin (CD103), in conjunction with the CD45RA, has improved discrimination between naive and memory T cells.22,23 However, similarly to CD45RA, none of these surface markers are specific for RTEs. Therefore, although highly helpful for the assessment of T-cell reconstitution, immunophenotyping also has some limitations in the assessment of thymopoiesis.
A more accurate quantification of thymic RTE output is provided by the measurement of T-cell receptor excision circles (TRECs).24 These are circular molecules of extrachromosomal DNA and represent by-products of the TCR recombination events that take place during intrathymic T-cell development25 (Figure 2). Measurement of signal joint TRECs (sjTRECs) with quantitative competitive (QC)–polymerase chain reaction (PCR) has been extensively used for the assessment of thymopoiesis and RTE output.24,25 Although useful in monitoring thymopoietic recovery after HSCT, the sjTREC assay may be influenced by peripheral T-cell proliferation because TRECs do not replicate with mitosis and are progressively diluted out with each division cycle. Thus, the dependency of TREC levels on the proliferative history of the peripheral T-cell pool is a limiting factor for comparisons. Measuring TREC levels per volume of blood rather than per nanogram of DNA can attenuate this effect. Similarly, the determination of the sj/β TREC ratio (termed thymic factor [TF]) has been introduced as a more accurate method for the assessment of thymopoiesis.26,27 The sj/β TREC ratio reflects the intensity of the intrathymic proliferation between the double negative (DN) and double positive (DP) stages, which is the major determinant of thymic RTE output. Because both sj and β TREC levels are equally diluted with subsequent cell divisions, their ratio is independent of peripheral cell proliferation and it is considered as an “RTE signature” of the peripheral T-cell compartment.
Molecular generation of TRECs during thymic differentiation of T cells. DN thymocytes first undergo rearrangement of the TCRB locus (encoding segments of the TCR-β chain). This begins with the rearrangement of the TCRBD to TCRBJ, which gives rise to several DβJβTRECs, and is followed by the recombination of V to DJ segments, which generates a greater variety of VDβTRECs (A). The TCRA locus is rearranged next, which, similarly to the β-chain, is characterized by enormous diversity. However, a common requirement for productive TCRAVJ recombination is deletion of the TCRD locus that it encompasses. This 2-step process gives rise to a signal joint TREC and a coding joint TREC (B). Both DNA families of TRECs are stable and do not replicate during mitosis. These sequences are unique to naive αβ T cells. As a result, TRECs serve as a valuable marker of RTEs and their levels are indicative of thymic activity.
Molecular generation of TRECs during thymic differentiation of T cells. DN thymocytes first undergo rearrangement of the TCRB locus (encoding segments of the TCR-β chain). This begins with the rearrangement of the TCRBD to TCRBJ, which gives rise to several DβJβTRECs, and is followed by the recombination of V to DJ segments, which generates a greater variety of VDβTRECs (A). The TCRA locus is rearranged next, which, similarly to the β-chain, is characterized by enormous diversity. However, a common requirement for productive TCRAVJ recombination is deletion of the TCRD locus that it encompasses. This 2-step process gives rise to a signal joint TREC and a coding joint TREC (B). Both DNA families of TRECs are stable and do not replicate during mitosis. These sequences are unique to naive αβ T cells. As a result, TRECs serve as a valuable marker of RTEs and their levels are indicative of thymic activity.
The diversity of T-cell repertoire, as assessed by CDR3 spectratyping or sequencing of the TCR β-chain, can also provide indirect information about thymic activity. T-cell diversity is almost exclusively accounted for by the naive population of lymphocytes. Consequently, TCR repertoire diversity after HSCT reflects the extent of naive T-cell production by the thymus and correlates with TREC levels.28
Thymic reconstitution after UCBT
Outcomes of single- and double-unit UCBT
The assessment of thymic function by measuring RTEs, TRECs, or TCR repertoire diversity has provided valuable insight into the kinetics and factors that affect thymic reconstitution after UCBT (Table 1). Studies of lymphocyte reconstitution in pediatric UCBT recipients have shown that despite a transient delay in the first few months, long-term lymphocyte recovery after single-unit UCBT (sUCBT) is comparable to matched sibling or unrelated HSCT recipients.29-31 These observations suggest a relative deficiency of the thymus-independent pathway of T-cell reconstitution after UCBT, which might be related to the low number and biologic properties of UCB T cells, but efficient support of T-cell recovery via the thymus-dependent pathway within the first year after UCBT. In such pediatric UCBT recipients, sjTREC and βTREC values reach a nadir at 3 months but recover to near pre-UCBT levels at 6 months after transplantation.32 By 1 year, recipients attain normal levels of sjTRECs,33 with a concomitant increase in naive CD4+ T-cell counts and TCR repertoire diversity.34,35 No significant differences in the timeline of thymic recovery have been observed compared with haploidentical or matched sibling HSCT recipients.32,35 In fact, at 2 years after transplantation, TREC values and TCR diversity were higher in UCBT recipients than in recipients of matched sibling donor grafts.35 These observations raise the intriguing hypothesis that LMPs of the UCB might have a superior potential to reconstitute thymopoiesis and TCR diversity compared with other HSC graft sources.
Published studies reporting findings on thymic reconstitution after UCBT
| Reference . | Patients (Median age, y) . | Conditioning and no. of UCB units . | TRECs . |
|---|---|---|---|
| 32 | UCBT: 24 pediatric patients (7.7) | MAC, no ATG, 1 UCB unit | No significant differences between the 2 groups. sjTRECs and βTRECs dropped at 3 mo and returned to ∼ pretransplant levels at 6 mo |
| Haplo HSCT: 33 pediatric patients (4.7) | |||
| 33 | 10 adult patients (29.3) | Variable, ATG, 1 UCB unit | 7 of 10 adult patients had detectable sjTRECs at 18 mo after UCBT, with median 325 copies per μg DNA at a median of 36 mo. All pediatric patients had detectable sjTRECs at 12 mo (earliest time point tested), median 6741 copies per μg DNA at median of 30 mo |
| 8 pediatric patients (4.6) | |||
| 34 | 30 pediatric patients (1) | No ATG, 1 UCB unit | TRECs detectable at 3 mo and substantially increased at 6-12 mo |
| 35 | 29 patients: | Variable ATG, 1 UCB unit | Normal levels at 1 y; comparable between UCBT and BMT groups. At 2 y, UCBT recipients had statistically higher TRECs than BMT recipients and young adults |
| UCBT: 10 patients (12.6) | |||
| MSD BMT: 19 patients (15) | |||
| 13 | 32 patients (33.5) | Variable, ATG, 1 UCB unit | 6 of 21 patients had detectable sjTRECs at baseline; 24 of 26 had undetectable sjTRECs during first year after UCBT |
| 40 | dUCBT: 29 patients (36) | MAC without ATG, 2 UCB units | TRECs lower in dUCBT recipients at 3 mo vs MRD/MUD group, but comparable in the 2 groups by 6 mo. Levels remained lower than normal in both groups at 12 mo |
| MSD BMT: 33 patients (45) | |||
| Matched unrelated: 33 patients (41) | |||
| 41 | 27 patients (48) | RIC, ATG, 2 UCB units | All evaluable patients had detectable sjTRECs at baseline; 3 of 10 at day 100; 10 of 15 at 6 mo (median 117 copies per μg DNA); 14 of 15 at 1 y (median 2136 copies per μg DNA) |
| 42 | 13 patients (42) | MAC without ATG (9 patients) and RIC + ATG (4 patients), + post-UCBT PTH, 2 UCB units | All patients had detectable sjTRECs at baseline; 3 of 7 at day 100; 4 of 6 at 6 mo (median 442 copies per μg DNA); all patients alive at 1 and 2 y had normal (>2000 copies per μg DNA) levels |
| Reference . | Patients (Median age, y) . | Conditioning and no. of UCB units . | TRECs . |
|---|---|---|---|
| 32 | UCBT: 24 pediatric patients (7.7) | MAC, no ATG, 1 UCB unit | No significant differences between the 2 groups. sjTRECs and βTRECs dropped at 3 mo and returned to ∼ pretransplant levels at 6 mo |
| Haplo HSCT: 33 pediatric patients (4.7) | |||
| 33 | 10 adult patients (29.3) | Variable, ATG, 1 UCB unit | 7 of 10 adult patients had detectable sjTRECs at 18 mo after UCBT, with median 325 copies per μg DNA at a median of 36 mo. All pediatric patients had detectable sjTRECs at 12 mo (earliest time point tested), median 6741 copies per μg DNA at median of 30 mo |
| 8 pediatric patients (4.6) | |||
| 34 | 30 pediatric patients (1) | No ATG, 1 UCB unit | TRECs detectable at 3 mo and substantially increased at 6-12 mo |
| 35 | 29 patients: | Variable ATG, 1 UCB unit | Normal levels at 1 y; comparable between UCBT and BMT groups. At 2 y, UCBT recipients had statistically higher TRECs than BMT recipients and young adults |
| UCBT: 10 patients (12.6) | |||
| MSD BMT: 19 patients (15) | |||
| 13 | 32 patients (33.5) | Variable, ATG, 1 UCB unit | 6 of 21 patients had detectable sjTRECs at baseline; 24 of 26 had undetectable sjTRECs during first year after UCBT |
| 40 | dUCBT: 29 patients (36) | MAC without ATG, 2 UCB units | TRECs lower in dUCBT recipients at 3 mo vs MRD/MUD group, but comparable in the 2 groups by 6 mo. Levels remained lower than normal in both groups at 12 mo |
| MSD BMT: 33 patients (45) | |||
| Matched unrelated: 33 patients (41) | |||
| 41 | 27 patients (48) | RIC, ATG, 2 UCB units | All evaluable patients had detectable sjTRECs at baseline; 3 of 10 at day 100; 10 of 15 at 6 mo (median 117 copies per μg DNA); 14 of 15 at 1 y (median 2136 copies per μg DNA) |
| 42 | 13 patients (42) | MAC without ATG (9 patients) and RIC + ATG (4 patients), + post-UCBT PTH, 2 UCB units | All patients had detectable sjTRECs at baseline; 3 of 7 at day 100; 4 of 6 at 6 mo (median 442 copies per μg DNA); all patients alive at 1 and 2 y had normal (>2000 copies per μg DNA) levels |
ATG, anti-thymocyte globulin; BMT, bone marrow transplantation; dUCBT, double-unit UCBT; haplo, haploidentical; HSCT, hematopoietic stem cell transplantation; MAC, myeloablative conditioning; MRD, matched related donor; MSD, matched sibling donor; MUD, matched unrelated donor; PTH, parathyroid hormone; RIC, reduced-intensity conditioning; sjTREC, signal joint TREC; TREC, T-cell receptor excision circle; UCB, umbilical cord blood; UCBT, UCB transplantation.
In striking contrast to these findings in pediatric patients who undergo sUCBT, a marked delay in thymic recovery has been observed after sUCBT in adults. Komanduri et al studied immune reconstitution parameters in a cohort of 32 patients undergoing sUCBT.13 These patients were heavily pretreated, had impaired thymopoiesis at baseline, and received various conditioning regimens, with the inclusion of anti-thymocyte globulin (ATG). During the first year, patients displayed near complete absence of sjTRECs, paucity of naive T cells, and late memory T-cell skewing. Another study of sUCBT in adult recipients, reported TREC values below the age-adjusted normal controls, even at 36 months after transplantation.33 Whether this marked difference in the kinetics of thymic recovery between pediatric and adult sUCBT recipients is primarily related to age or to nucleated cell dose remains unclear.
Double-unit UCBT (dUCBT) has been used to circumvent the cell dose limitation in adult patients.36,37 Compared with sUCBT, dUCBT results in earlier thymic recovery. In a prospective analysis from Dana-Farber/Harvard Cancer Center,38 CD4 counts were significantly lower in the dUCBT recipients compared with MUD transplant recipients after reduced-intensity conditioning (RIC) for 6 months, but by 12 months the CD4 T-cell recovery was similar in the dUCBT and MUD cohort and reached normal levels by 24 months. Interestingly, the recovery of naive CD4 cells (CD4+CD45RO−) in the dUCBT cohort was delayed in the first 6 months, but by 24 months it reached normal levels and significantly surpassed recovery in the MUD cohort.38 In a different report,39 paucity of naive (CD45RA+CCR7+) T cells in dUCBT recipients was observed during the first 6 months, followed by a gradual increase to levels comparable to those of healthy controls by 1 year. Thymopoiesis, as assessed by TREC levels, was also impaired until 9 months after transplantation, when a substantial increase of RTEs was observed. A more detailed comparison of immune reconstitution between adult dUCBT and matched sibling donor (MSD) or MUD transplant recipients after myeloablative conditioning (MAC) without ATG has been reported by Kanda et al.40 The RTE (CD4+CD45+CD62L+) count was significantly lower in dUCBT recipients during the first 6 months after transplantation but this difference disappeared at 1 year. TREC levels were also lower in dUCBT recipients at 3 months but this difference normalized between the 2 cohorts at 6 months and TCRβ repertoire was comparable by 12 months after transplantation.
In accord with those reports and in contrast to the protracted thymic deficiency observed after sUCBT, our group has shown early detection of TREC levels in adult dUCBT recipients both after RIC41 and MAC42 regimens. In a cohort of 27 adult patients who received identical RIC regimen with ATG, median sjTREC levels at the time of transplantation were slightly below normal range in this heavily pretreated population, fell dramatically after conditioning, and remained universally low through 100 days. However, at 6 months, 10 of 15 evaluable patients had detectable thymopoiesis and median TREC values reached normal limits by 1 year after dUCBT. In our second study,42 we analyzed 13 patients who enrolled in a phase 2 trial of dUCBT; 9 patients received MAC and 4 received RIC. Thymic reconstitution compared favorably to our previous study. TREC levels were detected as early as 100 days after dUCBT in a minority of patients and 67% of patients had detectable levels at 6 months. At 1 and 2 years, all surviving patients had normal levels of TRECs. The younger age of MAC recipients, omission of ATG in the MAC arm, and higher TREC levels at the time of transplantation in this cohort may have accounted for those differences.
Recently, Memorial Sloan Kettering Cancer Center (MSKCC) used a deep-sequencing analysis approach to measure TCR diversity with high resolution in 27 patients after conventional or T-cell depleted (TCD) peripheral blood stem cell (PBSC) transplantation or dUCBT.43 Interestingly, dUCBT recipients had the highest TCR diversity of all patients. The difference was more pronounced in the CD4+ T-cell diversity between dUCBT and TCD recipients after 6 months and was associated with a greater fraction of naive CD4+ T cells in dUCBT patients.
Taken together, these studies indicate that dUCBT supports improved quantitative and qualitative thymic recovery, compared with sUCBT. Although this might be related to a cell dose effect resulting in higher LMP numbers in the context of 2 UCB units, the majority of dUCBT recipients display hematopoietic reconstitution from 1 of the 2 UCB units well before the recovery of thymic function.37,44 This observation suggests that as-yet-unidentified mechanisms, other than cell dose-related HSC engraftment, might be involved in the improved thymic reconstitution after dUCBT.
Other factors affecting thymic reconstitution after UCBT
Several factors related to the characteristics of the host and the peritransplant clinical conditions have clinically important impact on the reconstitution of thymic function after allogeneic transplantation.26 Advanced recipient age has been associated with a delay in the recovery of TRECs and naive T cells, resulting in higher risk for opportunistic infections and inferior overall survival (OS).45,46 Similarly, in the setting of sUCBT, adult sUCBT recipients have markedly delayed thymic recovery compared with pediatric patients. Although one may consider that the lower cell dose per kilogram noted in adult sUCBT recipients may account for the inferior outcomes compared with pediatric populations, a multivariable analysis showed that recipient age and nucleated cell dose were independent prognostic factors in the setting of UCBT.47 The thymus is also a sensitive target of GVHD, which affects both its lymphoid and epithelial/stromal compartments. Features of “thymic GVHD” include thymocyte depletion, changes in the number and composition of thymic epithelial cells (TECs), disappearance of the corticomedullary demarcation, and absence of Hassall bodies. The distortion of normal architecture results in defective thymopoiesis.17 From a clinical standpoint, GVHD, both acute and chronic, has been identified as an independent determinant that impacts thymic recovery after HSCT or UCBT, and is associated with reduced naive T cells and TRECs and an oligoclonal T-cell repertoire.45,47,48 Importantly, immunosuppressive medications to prevent or treat GVHD can have detrimental effects on thymopoiesis.49,50 However, one study has suggested successful recovery of TRECs in a cohort of UCB recipients who received prophylactic immunosuppression in the absence of GVHD.48 An additional factor that can affect thymic reconstitution is related to the intensity of conditioning regimen. Cytotoxic chemotherapy and radiation have a negative impact on thymic function and RTE output.51,52 Theoretically, RIC regimens may cause less damage to the thymus resulting in faster regeneration of naive T cells. As an example, a multivariate analysis showed that the intensity of conditioning was the most important factor influencing TREC and CD4+ naive T-cell counts in the first 6 months after HSCT.53 In the same regard, Chao et al54 reported that patients undergoing sUCBT after RIC developed faster recovery of naive CD45RA+ T cells and TREC levels and more diverse T-cell repertoire compared with patients receiving sUCBT after MAC using identical ATG dose, GVHD prophylaxis, and supportive care.33 In another UCBT study, RIC was an independent factor for TREC recovery after UCBT in a multivariate model.47 Other factors, such as use of ATG,46 radiation, and pretransplant host TREC levels55 have also been implicated in the thymic recovery after transplantation. It is of note that due to the variable effect of the above factors, conclusions are not always consistent among different studies.
Clinically prognostic value of thymopoietic recovery after UCBT
UCBT is associated with increased rates of opportunistic infections compared with other graft sources, especially from viral pathogens that require intact T-cell immunity such as herpesviruses, adenovirus, and BK virus.12,56-60 Cytomegalovirus (CMV) is the most frequent opportunistic pathogen thought to contribute significantly to HSCT morbidity and mortality.61 The CMV source after UCBT is almost exclusively of host origin because the incidence of congenital CMV infection is very low.62 The frequency of CMV reactivation after UCBT is variable between studies, depending on patient characteristics and pretransplant seropositivity status. In contrast, the risk of CMV infection in UCB recipients is not associated with donor serology, which reflects the maternal exposure history rather than active or latent infection. However, UCB lacks CMV-specific memory cells that would confer adoptive immunoprotection against CMV, and this might have significant implications in the clinical outcome of CMV reactivation in UCBT recipients. In the largest published series of 332 UCBT recipients from the University of Minnesota,59 CMV reactivation was observed in 51% of seropositive subjects, which is comparable to the rates observed in recipients of adult HSCT. However, a relatively high 27.1% of patients experiencing reactivation developed clinical CMV disease, resulting in higher TRM and reduced OS. In another report of 330 pediatric UCBT recipients from Duke University,12 CMV was the second most common cause of infectious-related deaths in the first 6 months after transplantation. Another study from Japan reported CMV antigenemia in 79% of adult UCBT recipients, who were also more likely to require repeated courses of preemptive gancyclovir therapy compared with recipients of HSCT from adult donors.63 These findings suggest a delayed recovery of CMV-specific immunity after UCBT.
To determine the immunological mechanisms that lead to the restoration of functional CMV-specific immune responses, our group has examined parameters of immune reconstitution in the context of CMV reactivation in a cohort of 27 adult dUCBT recipients. CMV-specific effectors were detected by interferon-γ (IFN-γ) enzyme-linked immunospot assay as early as 8 weeks posttransplant, before the recovery of thymopoiesis, as evidenced by undetectable TREC levels at this early time point.41 Similarly, McGoldrick et al were able to detect IFN-γ+ CMV-specific CD4+ and CD8+ T cells of UCB origin after in vitro stimulation in the majority of seropositive patients in the first 56 days after transplantation.64 These findings suggest that both CD8+ and CD4+ UCB-derived naive T cells are primed to CMV early after UCBT and can give rise to CMV effectors, independently of thymic recovery. However, the UCB-derived CD8+ CMV-specific T cells remain at low numbers and fail to control CMV reactivation in the early posttransplant period.64 The functional deficiency of CMV-specific CD8+ T cells may be explained by the profound paucity of CD4+ T helper cells after UCBT,41,64 which are imperative for the development of a functional CD8+ T-cell response.65 This conclusion is further supported by the fact that in our study, clearance of CMV viremia was increasingly observed after 6 months and significantly correlated with the recovery of naive CD4+CD45RA+ T cells.41 Furthermore, clearance of CMV viremia was associated with the reemergence of TRECs, and UCBT recipients that attained normal TREC levels were more likely to display absence of CMV viremia, suggesting a critical contribution of the recovering thymopoiesis in the clinical control of the virus in vivo.41
The prognostic value of thymic recovery extends beyond its influence on reconstitution of pathogen-specific immunity. The COBLT study group has examined the clinical effect of the development of antigen-specific T-lymphocyte immunity against herpesviruses (herpes simplex virus [HSV], CMV, Epstein-Barr virus [EBV]) on outcomes in a cohort of 117 pediatric patients with acute myeloid leukemia (AML) or acute lymphocytic leukemia (ALL) who underwent UCBT.66 Recipients with a positive proliferative response against any herpesvirus in the first 3 years had lower infectious-related mortality67 but, more importantly, a markedly lower risk of leukemia relapse.66 Furthermore, recovery of thymic function has been correlated with decreased risk for leukemia relapse: in a combined analysis of 46 pediatric patients undergoing UCBT or haplo-HSCT, subjects who relapsed had significantly lower levels of sjTRECs or βTRECs before transplantation and during follow-up, at 3 and 6 months. In addition, lack of detectable sjTRECs and, more notably, βTREC levels, strongly correlated with increased incidence of relapse.32
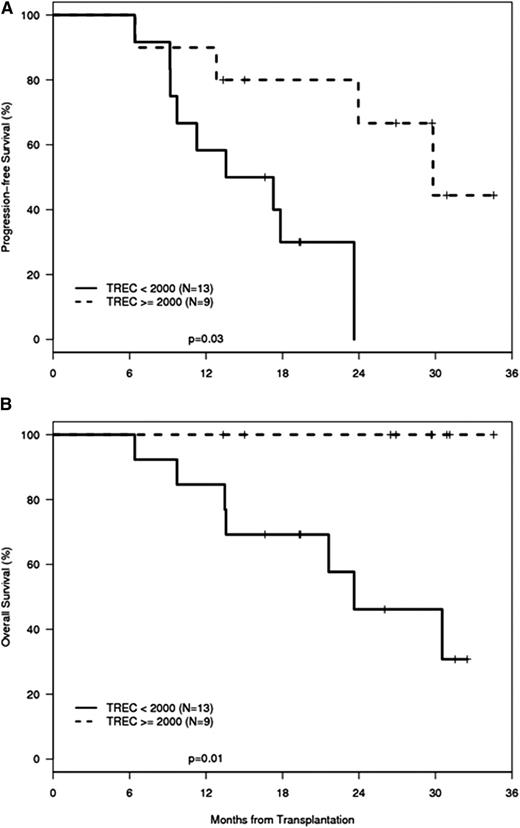
Findings in adult UCBT recipients are in accord with these observations in pediatric patients. Increased TREC levels displayed a strong correlation with attainment of CMV-specific immunity and absence of CMV viremia in adult dUCBT recipients.41 Because CMV immunity was used as a paradigm for successful immune reconstitution, this study also investigated whether reconstitution of CMV-specific immunity and parameters of cellular T-cell immunity may be directly linked to distinct outcomes of progression-free survival (PFS) and OS. Univariable and multivariable analysis demonstrated that improved OS and PFS were significantly associated with the ability of patients to develop CMV-specific responses. Given that reconstitution of functional CMV-specific immunity and absence of viremia were significantly associated with TREC recovery, it was hypothesized that the restoration of thymopoiesis might also imply a successful immune reconstitution and improved capacity for generation of immune responses against other pathogens or tumor antigens. Consistent with this hypothesis, the main causes of death in this patient cohort were relapse, posttransplant lymphoproliferative disorder (PTLD), and sepsis. Furthermore, assessment of OS showed that patients whose TREC levels were 2000 copies per μg DNA (the lowest limit of values range in healthy individuals) or more by 1 year after transplantation had significantly improved OS compared with patients whose TREC values remained <2000 copies per μg (Figure 3). Similarly to these findings, in a cohort of 50 patients who underwent UCBT at the Karolinska Institute, subjects with TREC levels above the median value at 6 months had a trend of increased OS.47 Although the relationship between TRECs and OS did not reach statistical significance, this observation is in accord with another report from the same institution showing a significant correlation between TREC levels and OS in adult patients who underwent HSCT from adult donors.46 Other studies have also reported the prognostic value of thymic recovery on clinical outcomes after adult HSCT.68,69 Taken together, these observations support the intriguing hypothesis that thymic differentiation of pathogen-specific and leukemia-specific T cells might occur in parallel in UCBT recipients and offer an opportunity to investigate further the kinetics and mechanisms of regeneration of such antigen-specific T-cell populations.
PFS and OS are higher in patients with regeneration of thymic function than in patients with impaired thymic regeneration after UCBT. A cohort of adult recipients of dUCBT treated with 1 protocol of pretransplant conditioning and posttransplantation immunosuppression41 was categorized into 2 groups: (1) patients with TREC values ≥2000 copies per μg DNA at 1 year after transplantation and (2) patients with TREC values <2000 copies per μg DNA at 1 year after transplantation. Kaplan-Meier estimates of PFS and OS were calculated as a function of the maximum TREC concentration attained at 1 year. Patients with TREC levels exceeding 2000 copies per μg DNA had a significantly improved PFS (P = .01) (A) and OS (P = .03) (B) compared with patients with TREC levels <2000 copies per μg DNA (n = 22).
PFS and OS are higher in patients with regeneration of thymic function than in patients with impaired thymic regeneration after UCBT. A cohort of adult recipients of dUCBT treated with 1 protocol of pretransplant conditioning and posttransplantation immunosuppression41 was categorized into 2 groups: (1) patients with TREC values ≥2000 copies per μg DNA at 1 year after transplantation and (2) patients with TREC values <2000 copies per μg DNA at 1 year after transplantation. Kaplan-Meier estimates of PFS and OS were calculated as a function of the maximum TREC concentration attained at 1 year. Patients with TREC levels exceeding 2000 copies per μg DNA had a significantly improved PFS (P = .01) (A) and OS (P = .03) (B) compared with patients with TREC levels <2000 copies per μg DNA (n = 22).
It has been described that dUCBT is associated with lower rates of leukemia relapse compared with sUCBT70 or HSCT from adult related or unrelated donors.71,72 This compensates for the higher treatment related mortality (TRM) at earlier time points, resulting in comparable long-term survival between dUCBT and HSCT recipients from adult donors. Although the mechanisms for reduced leukemia relapse in dUCBT recipients have not been identified, intriguing studies from MSKCC showed that dUCBT recipients have superior TCR repertoire diversity at 6 and 12 months after transplantation compared with patients undergoing HSCT from adult donor grafts, with or without T-cell depletion.43 Considering the essential role of the thymus for the diversification of TCR repertoire and the prompt recovery of TRECs after dUCBT,41 these observations provide indirect evidence that enhanced thymopoiesis might lead to the development of T cells with a broad TCR repertoire, including clones specific for graft-versus-leukemia (GVL), which account for the decreased relapse risk in dUCBT recipients.
These extensive studies provide compelling evidence that recovery of thymopoiesis plays a critical role in the generation of competent immune responses against viral or tumor antigens both in pediatric and adult UCBT recipients. Therefore, assessment of thymic reconstitution might be a valuable predictive factor of clinical outcomes after UCBT.
Approaches to improve thymic regeneration and function after HSCT
The use of dUCBT is currently the standard of care for adult patients in most US centers and is associated with improved myeloid engraftment and reduced risk of graft failure.73 Moreover, studies suggest that dUCBT recipients may also display faster thymic reconstitution compared with sUCBT recipients.40-42 This outcome may be related to a cell dose effect, with higher numbers of LMPs infused in dUCBT recipients leading to more efficient thymic seeding. However, alternative yet unidentified mechanisms may also be involved, considering that the majority of patients demonstrate single-unit chimerism by 3 months,37,44 before the emergence of RTEs).
Cytokine-based approaches
Experimental approaches to improve thymopoietic recovery after HSCT are under investigation in preclinical models or human clinical trials, although they have not been tested specifically in the setting of UCBT. KGF is an important trophic factor for TECs.74 In several murine models of HSCT, exogenous KGF administration has been shown to protect TECs from chemotherapy, radiation, or GVHD-induced damage, leading to increased production of intrathymic IL-7 and enhanced thymopoiesis.75 However, in human trials, administration of KGF did not show a beneficial effect on immune recovery.76
Administration of IL-7 in mice has shown not only to enhance thymic reconstitution, but also has a beneficial effect on the thymic-independent pathway of T-cell regeneration by mediating survival and proliferation of naive T cells.77,78 The immunorestorative effects of IL-7 have similarly been observed in primates and, more recently, in clinical trials in humans.79 However, studies have not provided definitive evidence for augmented thymopoiesis in humans. Patients with advanced malignancies80 or HIV81 treated with exogenous recombinant human IL-7 (rhIL-7) displayed a sustained expansion of T cells, preferentially of the naive subsets, higher TRECs, and a marked increase in the TCR repertoire diversity. On the other hand, no increase in thymic size was noted by positron emission tomography/computed tomography scanning, suggesting that IL-7 may primarily work at a postthymic level by enhancing proliferation of naive T cells including RTEs.80 In the same regard, it is important to note that IL-7 plays an important role in the trafficking of RTEs among peripheral blood and secondary lymphoid orgrans,82 possibly confounding the use of TREC assays as markers of thymic output. Recently, rhIL-7 was tested in a phase 1 trial of 12 patients undergoing T-cell-depleted HSCT.83 This resulted in significant expansion of effector memory T cells, which was associated with an increase in virus-specific T cells and enhanced TCR repertoire. However, no effect on TREC levels and RTE output was observed, at least at the tested IL-7 dose and follow-up period of the study.
In a murine model of HSCT, treatment with Fms-related tyrosine kinase 3 ligand (FLT3L) resulted in significantly increased numbers of thymic-dependent progeny and TREC+ T cells, suggesting increased thymopoiesis.84 However, although FLT3L increased thymic output in peripheral organs, the thymus itself was not evaluated in that study. Thus, it remains equivocal whether thymopoiesis was actually increased or this observation is due to pre- or postthymic mechanisms, such as thymic homing of LMPs or RTE trafficking, especially considering that FLT3L has potent effects on hematopoietic stem cells and also enhances the thymic-independent pathway of peripheral T-cell expansion.84,85 Sex steroid ablation with luteinizing hormone-releasing hormone analogue (LHRH-A) before HSCT in mice is associated with increased numbers of myeloid and lymphoid progenitors in the BM and improved T-cell recovery.86 In humans, LHRH-A administration prior to HSCT has been shown to lead to faster recovery of total and naive TREC+CD4+ T cells and diversification of T-cell repertoire, suggesting an effect of sex hormone blockade on thymic recovery.87 In a mouse model of radiation-induced thymic injury, it was determined that depletion of DP thymocytes triggered intrathymic production of IL-22,88 which appeared to have an active role in thymic recovery by promoting survival and proliferation of TECs. Importantly, systemic administration of IL-22 enhanced thymopoiesis following total body irradiation. Whether the above approaches are applicable to UCBT remains to be determined.
Cell-based approaches
To enhance HSC engraftment of the UCB, several groups have explored various strategies of ex vivo expansion of the UCB.89 Based on preclinical observations that the Notch signaling pathway plays a critical role in the regulation of self-renewal and repopulating ability of HSC progenitors of the UCB90,91 as well as fate decision of HSCs toward T-cell differentiation,92 Delaney et al have developed an ex vivo culture system for UCB progenitors based on immobilized Notch ligand Delta 1.93 However, in a phase 1 trial of dUCBT using 1 ex vivo–expanded and 1 unmanipulated unit, although a transient multilog increase in the ex vivo–expanded CD34+ and total nucleated cells (TNCs) was observed, long-term hematopoietic reconstitution was achieved by the nonexpanded unit. Similarly to the effects of ex vivo expansion via Notch-mediated signaling, ex vivo coculture of UCB mononuclear cells with mesenchymal cells resulted in a rapid increase of neutrophil numbers after dUCBT.94 However, this approach also induced only transient chimerism from the ex vivo–manipulated UCB, which eventually declined, resulting in long-term engraftment and hematopoiesis by the unmanipulated UCB in all patients. No effects on the reconstitution of adaptive immunity were noted by any of these cell-based approaches.
In an effort to selectively improve thymic dysfunction and T-cell reconstitution after HSCT, recent studies discovered that human progenitor T cells, developed in vitro from UCB stem cells with the use of OP9-DL1 culture system, could be a source of thymus-seeding progenitors when infused in NSG mice. Detailed cellular analysis revealed that between 2 distinct in vitro–derived progenitor human T-cell populations (proT1 and proT2), proT2 cells (CD34+CD45RA+CD7++) had superior engrafting capacity in the recipient thymus. More importantly, these in vitro–derived proT2 cells could also restore thymic architecture and when coinfused with HSCs, were able to promote HSC-derived T-cell lymphopoiesis.95 These findings suggest that Notch-based ex vivo culture of human progenitor T cells might represent a clinically applicable novel method to support T-cell reconstitution in UCBT recipients.
Based on previous observations that prostaglandin E2 (PGE2) is a critical regulator of HSC homeostasis,96 Dana-Farber Harvard Cancer Center conducted a phase 1 trial of dUCBT using 1 ex vivo PGE2-treated UCB unit and 1 unmanipulated unit.97 This study showed enhanced neutrophil recovery in comparison with historic controls and, more importantly, long-term dominance of the PGE2-treated unit. Besides the effect on myeloid engraftment, this study also determined that PGE2 promoted the survival of UCB T cells via the Wnt/β-catenin pathway.98 In a different experimental system it was previously determined that Wnt signals mediate proliferation and cell adhesion, but not differentiation of immature thymocytes.99 Therefore, modulation of the PGE2/Wnt axis might also have a beneficial effect on thymopoiesis. In support of this hypothesis, a preliminary immune reconstitution analysis showed less TCR clonality in patients with PGE2-treated UCB unit dominance compared with historic control dUCBT recipients and patients with dominance of the non-PGE2-treated unit.100 Although preliminary, these findings suggest that PGE2 treatment might alter the properties of UCB-derived thymocyte progenitors, including survival, thymus colonization, and expansion, leading to enhanced thymopoiesis.
Summary and future directions
UCB is a valuable HSC source for patients requiring allogeneic transplantation who lack suitable sibling or unrelated adult donors. The distinct biologic properties of UCB grafts allow for a greater degree of HLA disparity and lead to lower rates of GVHD in UCBT recipients, without compromising the GVL effect. However, UCBT is associated with delayed engraftment and immune reconstitution, resulting in higher rates of early posttransplant infectious complications. Recovery of thymopoiesis is an imperative component of immune reconstitution after UCBT and plays an essential role in the restoration of peripheral T-cell compartment by the de novo production of naive lymphocytes with diverse TCR repertoire. Furthermore, parameters of thymic function have significant prognostic value for clinical outcomes in UCBT recipients. The use of double UCBT has lead to faster thymic regeneration in adult patients. Further strategies to improve thymic seeding, intrathymic proliferation, and differentiation of LMPs, or to protect the thymic microenvironment from the detrimental effects of conditioning and GVHD may further improve the outcomes of UCB transplantation.
Acknowledgments
This work was supported by the National Institutes of Health grants R56A1098129 and RO1CA183605, and by the HHV-6 Foundation pilot grant.
Authorship
Contribution: I.P. and V.A.B. wrote the paper.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Vassiliki A. Boussiotis, Beth Israel Deaconess Medical Center, 330 Brookline Ave, Dana 513-518, Boston MA 02215; e-mail: vboussio@bidmc.harvard.edu.