Key Points
Incidence of ITP was 2.9/100 000 person-years with age, seasonal, and regional variations; in adults, 18% were secondary.
Severe (gastrointestinal or central nervous system) bleeding at ITP onset was rare (<1%); the risk increased with age.
Abstract
The epidemiology of immune thrombocytopenia (ITP) is not well known. The purpose of this study was to assess ITP incidence at a nationwide level (France) with recent data (mid-2009 to mid-2011; 129 248 543 person-years). The data source is the French health insurance database. We selected cases with diagnosis codes for in-hospital stays and long-term disease attributions, thus restricting our search to ITPs necessitating health care. We studied incidence by age, gender, calendar month, regions, and proportion of secondary ITPs, of ITPs becoming persistent or chronic, and of severe bleeding at disease onset. We identified 3771 incident ITP patients. Incidence was 2.9/100 000 person-years, with peaks among children and in those >60 years of age. ITP was more frequent among males in these subgroups. The incidence was lower in overseas Caribbean French departments, suggesting a lower incidence among Afro-American people. There was a north-south gradient in mainland France and seasonal variations (peak in winter and nadir in summer). Persistence or chronicity occurred in 36% of children compared with 67% of adults. Among adults, 18% of ITPs were secondary. Malignancy was the main cause (10.9%). Myelodysplastic syndromes were not rare (2.3%). Severe gastrointestinal or central nervous system bleeding at ITP onset was rare (<1%).
Introduction
Immune thrombocytopenia (ITP) is a rare condition.1 It is due to a B-cell (and in some patients to a CD8+ T-cell) autoimmune reaction directed against circulating platelets and megakaryocytes, leading to life-threatening bleeding in some patients.2 Although this disease has been studied for more than a century,3 its epidemiology is not well known, and several points need to be clarified.
ITP incidence has been estimated to be from 0.46 to 12.5/100 000 person-years in children and from 1.6 to 3.9/100 000 person-years in adults.4 The largest epidemiologic study was conducted in the United Kingdom in the Clinical Practice Research Datalink (CPRD) that covers ∼3.4 million inhabitants: 1145 ITP cases were identified from 1990 to 2005, leading to an estimated overall incidence of 3.9/100 000 person-years, with a peak in older patients.5 The estimated incidence of pediatric ITP was 4.2/100 000 person-years.6 Incidence was increased in females except in children and older patients.5,6 Other population-based studies were underpowered to confirm these particularities.7
Various viruses may be environmental factors triggering ITP onset, particularly in pediatric ITP.8 However, seasonal variations of ITP incidence have not yet been studied in adults.
The incidence of ITP may be lower among black populations, but this point is still debated.9,10 Apart from ethnic particularities, geographical factors may affect the incidence of autoimmune diseases.11 The geographical variations of ITP incidence across a wide area has not yet been assessed.
ITP is said to be primary when it is not associated with another disease. Secondary ITP might be due to malignancy, systemic autoimmune disease, chronic viral infection such as HIV or hepatitis virus B or C (HCV), primary immune deficiency, or drugs. Expert opinion estimated that ∼20% of ITP cases are secondary,8 whereas only 8.7% of the patients had a comorbid condition associated with ITP in the CPRD study.5 This assessment deserves to be repeated due to changes in the epidemiology of diseases associated to ITP, such as myelodysplastic syndromes or HIV infection.12
The prevalence of severe bleeding at diagnosis is not well known. In a multinational ITP registry, gastrointestinal bleeding was observed in 2.9% of children and 1.2% of adults, and central nervous system (CNS) bleeding was observed in 0.6% and 1.8%, respectively. However, this registry stemmed from reference centers, and few adult patients (n = 340) were included.13 Bleeding risk might increase with age,14 but faithful assessment of such rare events needs larger cohorts.
On the basis of case series published from 1954 to 1990, the proportion of primary ITP leading to persistency (lasting >3 months) or chronicity (lasting >12 months)1 has been estimated as 20% in children and 70% in adult patients.15 This information has not been yet confirmed in any population-based study.
The objectives of the present work was to assess at a nationwide scale the overall incidence of ITP requiring health care, the incidence by age, gender, seasons, and across regions, the proportion of secondary ITP and of ITP leading to persistency, and the frequency of severe bleeding at presentation. Indeed, ITP requiring health care represents the population of interest at a public health point of view.
Methods
Data source
The data source is the Système National d'Information Inter-Regimes de l'Assurance Maladie (SNIIR-AM), a unique database of the French National Health Insurance System.16 This has been widely used to conduct large epidemiologic studies.17-23 Further information regarding its organization is provided in the supplemental Appendix available on the Blood Web site. The SNIIR-AM collects prospective data on demographic and health expenditure reimbursements of the entire French population (65 586 million inhabitants in January 2013). It includes data on hospital stays, outpatient drug reimbursements, procedures, examinations, and sick leaves. The medical indication for outpatient reimbursements is not available, except for patients with a costly long-term disabling disease (LTD), who are fully reimbursed for most of their disease-related expenditures. LTDs and hospital diagnosis codes are encoded with the International Classification of Diseases, version 10 (ICD-10). Demographic data include age, gender, town of residence, date of death, and insurance system. These data are individualized, anonymous, exhaustive, and linkable for a given patient.
SNIIR-AM data availability cannot excess 3 years prior to the date of extraction.24,25 Identification of ITP patients from the SNIIR-AM has received ethical approval as part of the French Adult Primary Immune Thrombocytopenia: A Pharmacoepidemiological Study (FAITH), an ongoing cohort of all primary ITP adult patients persistently treated in France from 2009 to 2022. FAITH is registered in the European Post-Authorization Safety studies registry of the European Network of Centres for Pharmacoepidemiology and Pharmacovilange (no. ENCEPP/SDPP/4574).26 FAITH methodology has been awarded the European Network of Centres for Pharmacoepidemiology and Pharmacovilange study seal of approval. All ethical authorizations have been obtained (Institut des Données de Santé approval, no. 40, March 2012; Commission Nationale de l’Informatique et des Libertés authorization, no. DE-2012-076, July 2012).
Selection process of incident ITP patients requiring health care
Incident ITP patients were identified among patients with hospitalization and/or LTD attribution for ITP. The selection process followed several steps. First, 2009 to 2011 data from patients with an LTD encoded as ITP (ICD-10 code D69.3) and/or ≥1 hospital stay with a main or related diagnosis encoded as D69.3 during this period were extracted from the SNIIR-AM.
Second, we dropped cases that may have been miscoded, that is, patients having an LTD or hospital diagnosis code starting with D69 but different from D69.3 (ITP), D69.6 (thrombocytopenia, unspecified), and D69.9 (hemorrhagic condition, unspecified) from study start (January 1, 2009) to 6 months after the occurrence of the first ITP code.
Third, we defined the date of diagnosis. For that purpose, we searched for out-hospital dispensing of ITP drugs (systemic steroids, dapsone, danazol, thrombopoietin receptor agonists, azathioprine, ciclosporin, mycophenolate)27,28 before the first LTD or hospital stay with the ITP code. Date of diagnosis was then defined as (1) the date of the first ITP drug dispensing if a patient had a persistent dispensing of ITP drugs before the first LTD or hospital stay with ITP code or (2) the first LTD or hospital stay with an ITP code. Persistent dispensing was defined as ≥3 consecutive dispensing within a 6-month period.
Last, we restricted the cohort to incident patients, excluding those with a date of diagnosis before July 1, 2009. Indeed, we could not assess whether a patient with a date of diagnosis during the first semester of 2009 was prevalent or incident, because we could not access data prior to January 1, 2009. We also excluded patients with a date of diagnosis after June 30, 2011, because we could not ascertain the absence of an erroneous D69 code during the semester following diagnosis. Therefore, the incident ITP cases included in this study occurred between July 1, 2009 and June 30, 2011, and the follow-up was ≥6 months for all cases.
Definitions
Adult patients were ≥18 years of age at the date of diagnosis. Secondary ITP patients were defined as patients having a new LTD code and/or hospital stay diagnosis code for a disease associated to ITP from the year before to the semester after the date of diagnosis (ICD-10 codes are detailed in Table 1). Evans syndrome was defined similarly, using the ICD-10 code D59.1 (autoimmune hemolytic disease). Persistent or chronic ITP was defined as an ITP LTD attribution, 2 hospital stays with ITP code ≥3 months apart, a continuous exposure to ITP drugs (including polyvalent intravenous immunoglobulins) for >3 consecutive months, or exposure to rituximab or splenectomy. Because some patients entering remission during the persistent phase of the disease may have relapsed during the chronic phase after the end of follow-up, it was not possible to accurately distinguish patients in complete remission at the end of the persistency phase from those who entered the chronic phase of the disease. However, this concerns a very low proportion of patients in practice.29
Causes of secondary ITP in adults
| Cause* . | ICD10 codes . | n . | Percent of secondary ITP (n = 518) . | Percent of ITP (n = 2882) . |
|---|---|---|---|---|
| In situ neoplasms | D00-D09 | 1 | 0.19% | 0.03% |
| Malignant neoplasms | C00-C97 | 314 | 60.62% | 10.89% |
| Hematological malignancies | C77, C81-C96 | 196 | 37.84% | 6.80% |
| Lymphoma | C77, C81-C86 | 91 | 17.57% | 3.16% |
| Hodgkin lymphoma | C81 | 16 | 3.09% | 0.55% |
| B-cell chronic lymphocytic leukemia | C91.1 | 49 | 9.46% | 1.70% |
| Multiple myeloma and malignant plasma cell neoplasms | C90 | 16 | 3.09% | 0.55% |
| Waldenström macroglobulinaemia | C88.0 | 13 | 2.51% | 0.45% |
| Myelodysplastic syndromes | D46 | 67 | 12.93% | 2.32% |
| Antiphospholipid syndrome | D68.6 | 8 | 1.54% | 0.28% |
| Viral hepatitis C or B | B16, B18.0-B18.2 | 9 | 1.74% | 0.31% |
| Viral hepatitis C | B18.2 | 5 | 0.96% | 0.17% |
| Viral hepatitis B | B16, B18.0-B18.1 | 4 | 0.77% | 0.14% |
| Human immunodeficiency virus disease | B20-B24 | 27 | 5.21% | 0.94% |
| Connective tissue disease | M32-M35.1 | 71 | 13.71% | 2.46% |
| Systemic lupus erythematosus | M32 | 49 | 9.45% | 1.70% |
| Systemic sclerosis | M34 | 3 | 0.58% | 0.10% |
| Dermatopolymyositis | M33 | 2 | 0.39% | 0.07% |
| Sicca syndrome | M35.0 | 16 | 3.09% | 0.55% |
| Mixed connective tissue disease | M35.1 | 3 | 0.58% | 0.10% |
| Rheumatoid arthritis | M05, M06.0, M06.2-M06.3, M06.8-M06.9 | 11 | 2.12% | 0.38% |
| Sarcoidosis | D86 | 18 | 3.47% | 0.62% |
| Immunodeficiency† | D80-D84 | 49 | 9.46% | 1.70% |
| Cause* . | ICD10 codes . | n . | Percent of secondary ITP (n = 518) . | Percent of ITP (n = 2882) . |
|---|---|---|---|---|
| In situ neoplasms | D00-D09 | 1 | 0.19% | 0.03% |
| Malignant neoplasms | C00-C97 | 314 | 60.62% | 10.89% |
| Hematological malignancies | C77, C81-C96 | 196 | 37.84% | 6.80% |
| Lymphoma | C77, C81-C86 | 91 | 17.57% | 3.16% |
| Hodgkin lymphoma | C81 | 16 | 3.09% | 0.55% |
| B-cell chronic lymphocytic leukemia | C91.1 | 49 | 9.46% | 1.70% |
| Multiple myeloma and malignant plasma cell neoplasms | C90 | 16 | 3.09% | 0.55% |
| Waldenström macroglobulinaemia | C88.0 | 13 | 2.51% | 0.45% |
| Myelodysplastic syndromes | D46 | 67 | 12.93% | 2.32% |
| Antiphospholipid syndrome | D68.6 | 8 | 1.54% | 0.28% |
| Viral hepatitis C or B | B16, B18.0-B18.2 | 9 | 1.74% | 0.31% |
| Viral hepatitis C | B18.2 | 5 | 0.96% | 0.17% |
| Viral hepatitis B | B16, B18.0-B18.1 | 4 | 0.77% | 0.14% |
| Human immunodeficiency virus disease | B20-B24 | 27 | 5.21% | 0.94% |
| Connective tissue disease | M32-M35.1 | 71 | 13.71% | 2.46% |
| Systemic lupus erythematosus | M32 | 49 | 9.45% | 1.70% |
| Systemic sclerosis | M34 | 3 | 0.58% | 0.10% |
| Dermatopolymyositis | M33 | 2 | 0.39% | 0.07% |
| Sicca syndrome | M35.0 | 16 | 3.09% | 0.55% |
| Mixed connective tissue disease | M35.1 | 3 | 0.58% | 0.10% |
| Rheumatoid arthritis | M05, M06.0, M06.2-M06.3, M06.8-M06.9 | 11 | 2.12% | 0.38% |
| Sarcoidosis | D86 | 18 | 3.47% | 0.62% |
| Immunodeficiency† | D80-D84 | 49 | 9.46% | 1.70% |
Causes of secondary ITP were searched from the year before and up to 6 months after ITP date of diagnosis.
Other than HIV infection. Sensitivity analysis excluding the codes possibly corresponding to secondary immunodeficiency: n = 25.
To assess severe bleedings at diagnosis, we assumed that all the patients with such severe bleeding were hospitalized. We searched the ICD-10 codes corresponding to gastrointestinal and CNS bleeding at first hospital stay for ITP, when its date corresponded to the date of diagnosis.
Statistical analyses
For incidence calculations, we used as the denominator the French population data edited by the Institut National de la Statistique et des Etudes Economiques.30 As there is no detailed data regarding ethnicity in France,31-33 we compared incidence of ITP in mainland France to the incidence in overseas French departments (Reunion Island and French departments of America: Guadeloupe, Martinique, and French Guyana) where black and mixed-race population prevalence is high.34-37 The Afro-American population is the main ethnic group in the Caribbean (Guadeloupe and Martinique) departments.35 We calculated overall incidence and its 95% confidence intervals (CIs), as well as incidence by age, gender, calendar months, and across the 22 mainland administrative regions. To detect variations of incidence across regions, we directly standardized incidence rates by age (by 10-year intervals) and gender characteristics of the entire French population and used the local indicators of spatial associations method.38 We searched for a relation between age groups and severe bleeding using a linear regression model (α = 5%).
Results
Patient selection
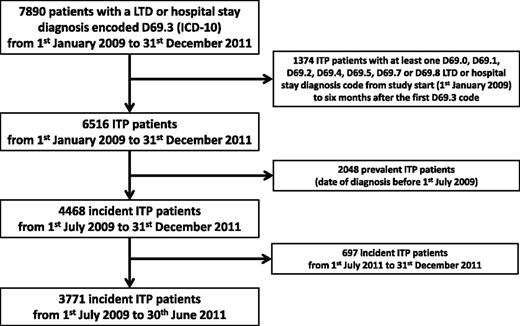
Of 7890 patients with an LTD or hospital stay diagnosis code D69.3 from January 1, 2009 to December 31, 2011, we identified 3771 incident ITP cases requiring health care during a 2-year period (Figure 1); 2885 (76.5%) were adults.
Flowchart illustrating patient selection. The date of diagnosis was refined for 478 patients who had ≥3 ITP drugs dispensed during a 6-month period before the first D69.3 code.
Flowchart illustrating patient selection. The date of diagnosis was refined for 478 patients who had ≥3 ITP drugs dispensed during a 6-month period before the first D69.3 code.
Incidence
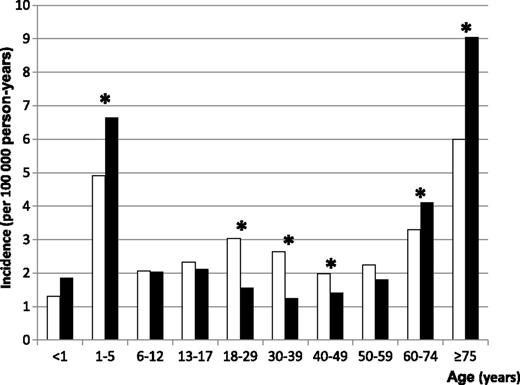
Overall ITP incidence was 2.92/100 000 person-years (95% CI: 2.83-3.01). It was higher in females (3.03/100 000 person-years, 95% CI: 2.90-3.16) than in males (2.77/100 000 person-years, 95% CI: 2.64-2.90). The incidence was 2.83/100 000 person-years in those <18 years of age (95% CI: 2.63-3.00) and 2.94/100 000 person-years in adults (95% CI: 2.84-3.05). A peak was observed in children aged 1 to 5 years, with the highest incidence in younger boys. A second peak was observed in adults >60 years of age, reaching 9/100 000 person-years (95% CI: 8.21-9.95) in men >75 years of age (Figure 2).
Incidence of ITP in France during the period from mid-2009 to mid-2011 by age and gender. Females, white bars; males, black bars. Stars indicate statistically significant differences among males and females (α = 5%).
Incidence of ITP in France during the period from mid-2009 to mid-2011 by age and gender. Females, white bars; males, black bars. Stars indicate statistically significant differences among males and females (α = 5%).
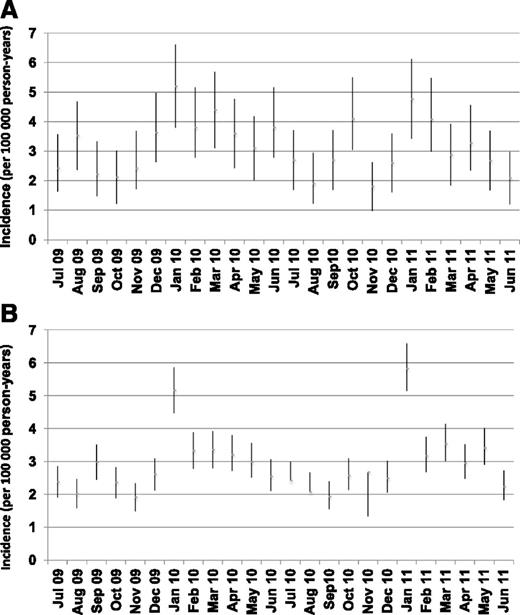
Incidence varied cyclically in the year, with a peak in January and a nadir in summer for both in adults and children (Figure 3). This finding was observed in all age groups except in the 0- to 1-year group (peak was in spring; supplemental Figure 1) and across regions (data not shown).
Variation of incidence of ITP in France during the period from mid-2009 to mid-2011 by calendar month. (A) Children <15 years of age and (B) adults ≥20 years of age.
Variation of incidence of ITP in France during the period from mid-2009 to mid-2011 by calendar month. (A) Children <15 years of age and (B) adults ≥20 years of age.
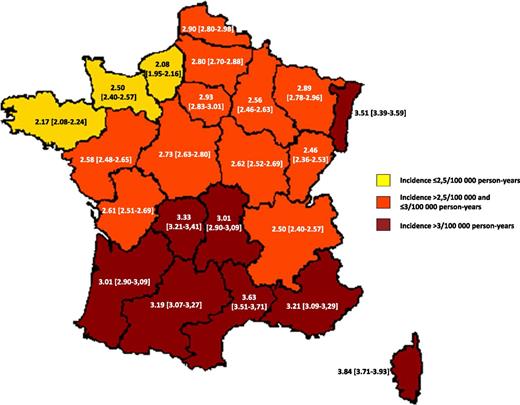
The incidence was higher in mainland France (2.93/100 000 person-years, 95% CI: 2.84-3.02) than in overseas French departments (1.45/100 000 person-years, 95% CI: 1.11-1.89). It was 1.14/100 000 person-years (95% CI: 0.72-1.79) in the Caribbean departments. Mean age at ITP onset was lower in the overseas departments in comparison with mainland France (median: 28 and 48 years, respectively; P = .005), whereas female gender (58.8% and 53.6%, respectively; P = .5) and frequency of severe bleeding at diagnosis (1.44% and 1.35%, respectively; P = 1) were similar.
Age- and gender-standardized incidence is mapped in Figure 4. There was no correlation with population density (data not shown). Local indicators of spatial associations analyses detected high incidence areas in west-northern France (centered by the Basse-Normandie and Pays de Loire regions) and low incidence areas in southern France (centered by the Midi-Pyrénées region) (P < .05).
Age- and gender-standardized incidence of ITP across administrative regions in mainland France. Direct standardization was made by age (by 10-year intervals) and gender in the general population in France.
Age- and gender-standardized incidence of ITP across administrative regions in mainland France. Direct standardization was made by age (by 10-year intervals) and gender in the general population in France.
Secondary ITP
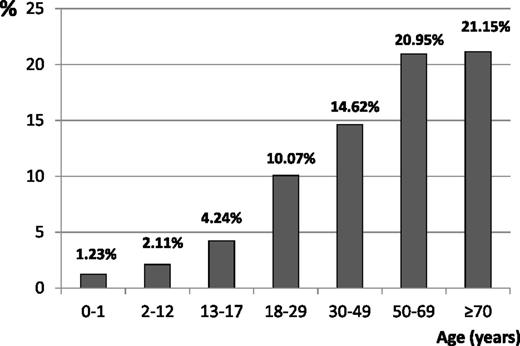
Eighteen percent of adult incident ITP patients requiring health care had secondary ITP (Table 1). Cancers (mainly hematological) were most frequent. Malignant lymphoid disorders were observed in 5.9% of incident adult ITPs. Other causes were connective tissue diseases (2.5%, mainly systemic lupus erythematosus), myelodysplastic syndromes (2.3%), immune deficiencies (excluding HIV infection, 1.7%), HIV infection (0.9%), sarcoidosis (0.6%), antiphospholipid syndrome (0.3%), and HCV infection (0.2%). Adult secondary ITP patients were older than primary ITP patients (mean ± standard deviation, 61.5 ± 18.8 vs 56.2 ± 22.0 years; P < .0001) and were predominantly males (48.4% vs 42.5%, P = .01), but they had a similar rate of severe bleeding at ITP onset (1.54% vs 1.61%, P = .9).
ITP was secondary in 2.4% of children cases. Causes were mainly primary immune deficiency, systemic lupus erythematosus, blood cancers, and HIV infection. Among children, secondary ITP patients were also older (median ± interquartile range, 7 ± 11 vs 5 ± 10) and were more frequently female (56.5% vs 36.8%).
The frequency of secondary ITP increased with age (Figure 5).
Forty-seven adult patients (1.63%) and 9 children (1.1%) had Evans syndrome.
Persistent or chronic primary ITP patients
With a mean follow-up of 17.6 ± 6.8 months (range, 6-30 months), 1556 (66.7%) of incident primary adult ITPs became persistent or chronic. Fifty-two percent of adult patients had a marker of the disease (ITP hospital diagnosis, LTD, or exposure to any ITP treatment) during the period M12 to M18 after the diagnosis and 47.8% in the M18 to M24 period. Among children, 276 (35.7%) incident primary ITP cases were persistent or chronic, with a mean follow-up of 17.9 ± 6.7 months (range, 6-30 months).
Major bleeding symptoms at diagnosis
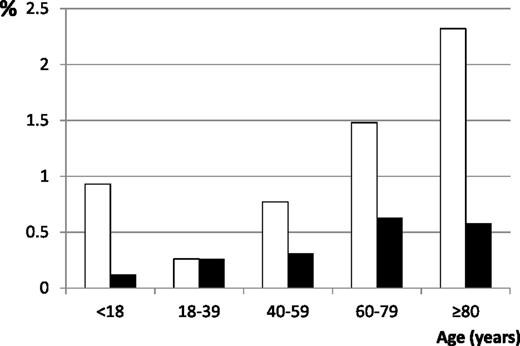
Diagnosis date corresponded to a hospital stay for 2899 (76.9%) incident patients. Gastrointestinal bleeding at diagnosis was found in 1.45% of hospitalized patients at diagnosis and CNS bleeding at diagnosis was found in 0.48%. Therefore, 1.11% of all patients presented with gastrointestinal bleeding and 0.37% with CNS bleeding. There was a linear increasing relation between age and gastrointestinal bleeding among adults and between age and CNS bleeding in the whole population (Figure 6). Adults with severe bleeding at ITP onset were older (median age: 73 vs 60 years, P = .01). There was no statistically significant difference regarding gender (males, 52.2% vs 43.4%, P = .2) and exposure to antiplatelet or anticoagulant drugs (10.9% vs 10.6%, P = 1). Among children, severe bleeding rates decreased with age: 1.3% in prepubertal patients and 0.6% in those >12 years of age.
Percentages of incident ITP patients with gastrointestinal (white bars) and CNS (black bars) bleeding by age. Linear testing for an increasing relation between age and severe bleeding at diagnosis was significant for gastrointestinal bleeding among adults (P = .003) and for CNS bleeding in the whole population (P = .02).
Percentages of incident ITP patients with gastrointestinal (white bars) and CNS (black bars) bleeding by age. Linear testing for an increasing relation between age and severe bleeding at diagnosis was significant for gastrointestinal bleeding among adults (P = .003) and for CNS bleeding in the whole population (P = .02).
Bleeding at diagnosis resulted in 5 deaths: 1 due to CNS bleeding at 73 years of age and 4 due to gastrointestinal bleeding (age, 82-85 years).
Discussion
This study estimated the incidence of ITP necessitating health care at a nationwide scale with recent data (2009-2011). Including 3771 incident patients in 2 years, it confirmed the age and gender characteristics of the CPRD study that included 1145 patients over 16 years,5 and it allowed calculation of incidence rates according to calendar months and regions. The completeness of the database allowed assessment of rare events such as rare disease associations and severe bleeding risk at ITP onset.
We found an overall incidence of 2.9/100 000 person-years (95% CI: 2.8-3.0), which is within the range of previous studies.4 It is lower than in the CPRD study (3.9/100 000 person-years, 95% CI, 3.7-4.1).5 The CPRD is supplied by general practitioners and therefore every diagnosis of ITP is recorded even if it is mild and not requiring treatment. Our study identified only incident ITP patients requiring health care for ITP, ie, those generating health expenditures. Therefore, our method underestimated the overall ITP incidence. Incidence of pediatric ITP is more debated, ranging from 0.46 to 12.5/100 000 person-years in previous studies.4 Once again, our estimate in children was lower than in the CPRD study, probably due to the same discrepancy.6
The increased incidence among patients ≥60 years of age was first suggested by Frederiksen et al.39 Schoonen et al refined these results in 2009, demonstrating the bimodal incidence with increased incidence among children and older patients.5 Our findings are fully in accordance with these data. The peak incidence in the patients 1 to 5 years of age has been previously suggested by the CPRD study and an international registry.6,40 We confirmed this finding by calculating incidences in the general population. We also confirmed the overall female predominance as well as male predominance in pediatric and older patients.5,41 In our study, the frequency of incident ITPs becoming persistent or chronic is also very close to experience- and smaller series-based findings for both adults and children.15,42,43 As previously said, we could not accurately measure the frequency of complete remission during the persistency phase before the chronic phase: in clinical practice, exposure to rituximab or splenectomy can occur early in the course of the disease when patients have a poor response to corticosteroids and/or polyvalent immunoglobulins. Both treatments have long-lasting effects and lead to prolonged remissions, allowing drug withdrawal in many cases, but relapses are not rare after some months or years. One can speculate that some patients would have cured spontaneously if not treated by rituximab or splenectomy. Moreover, patients entering in remission during the persistent phase of the disease may have relapsed during the chronic phase after the end of follow-up, and therefore, we could not accurately identify persistent from chronic ITP patients. Our study identified an increased ITP incidence in winter. One pediatric multinational registry assessed the role of seasonality in ITP and found an increased incidence in summer.40 Further studies are necessary to clarify this discrepancy. Our results add indirect argument to the role of viruses in ITP genesis. In children, two thirds of the patients experience flu-like fever during the weeks preceding ITP onset.8,44 The role of influenza virus has been suspected for a long time.44 It could play a direct role in ITP pathogenesis by promoting antiplatelet-antibodies production and/or decreasing platelet production. Infection may also prompt the request for a blood cell count, leading to a fortuitous discovery of thrombocytopenia. The pathogenic role of influenza vaccine is also debated, particularly in older patients.45-48 Further studies are ongoing in our cohort to study the impact of influenza vaccination on the occurrence of ITP.
For the first time, we also describe a gradient of incidence in mainland France, independent from age, gender, and population density. This suggests the role of unknown environmental factors in ITP pathogenesis. This should be confirmed by other studies across Europe using the same methodology. The lower incidence in overseas French departments (particularly Caribbean departments) compared with mainland France is a rough comparison among a dominant Afro-American and mixed-race population and an area with a dominant white population. It is unclear whether it is related to a different genetic background, to protective environmental factors, or to differences in the health care organization. In 2005, Terrell et al reviewed 7 studies and concluded that there were racial disparities.9 In 2006, a retrospective age-adjusted prevalence study carried out in US nationwide Veteran Affairs hospitals did not found any difference between whites and African Americans.10
Secondary ITP accounted for 18% of adult cases. This is very close to the experience-based 20% estimate by Cines et al.8 Nevertheless, malignancies (except B-cell chronic lymphocytic leukemia, 2%) were excluded from this latter assessment, whereas they are associated with 10.9% of ITPs in our cohort. Frequency of HIV infection (1%) and Evans syndrome (2%) are consistent. In the CPRD study, 8.7% of ITP patients had secondary ITP, but the diagnosis search was not as extensive as in our study, with the authors focusing their search mainly in the semester before and the semester after ITP diagnosis.5 Both hematological and solid cancers were more frequent in our cohort (6.80% vs 3.4% and 4.1% vs 0.7%, respectively). We do not explain these discrepancies. In contrast, the frequency of association with systemic lupus erythematosus was found to be very close to our findings (1.3%), as well as HCV infection (0.2%) and antiphospholipid syndrome (0.4%).5 Interestingly, we found an unexpected association with myelodysplastic syndromes (2.32% of ITP cases), as well as an association with neglected diseases in the context of ITP such as sarcoidosis (0.6%).49 However, ITP may have been misdiagnosed in some myelodysplastic syndrome patients, as this disease can be confused with ITP at an early stage.12
We found a very low risk of severe bleeding at diagnosis. As expected, it is lower in this population-based study than in the multinational registry including patients from reference centers, who may have a more severe disease.13 A higher risk of gastrointestinal bleeding was observed in children as previously suggested.13 We found a linear increase in the incidence of gastrointestinal bleeding among adults and of CNS bleeding in the whole cohort. The role of exposure to anticoagulant or antiaggregant in these events needs further investigation.14
The limitations of this study are inherent to all studies in administrative databases. We cannot rule out some misclassifications. However, we think that misclassification should not have concerned a significant proportion of patients. Indeed, (1) we dropped patients with probable erroneous codes (second step of the patient selection process); (2) 69.0% of incident patients received outpatient ITP drugs after the date of diagnosis, which is in accordance with previous data5 ; and (3) ICD diagnosis codes to identify ITP patients have been previously assessed in other administrative databases with a good predictive positive value.50,51
As quoted above, ITP incidence assessment among ethnic groups is quite difficult due to the lack of data in France.31,32 Because examination results are not recorded in the SNIIR-AM, we could not assess the role of some causes of secondary ITP such as Helicobacter pylori infection, and we could not check for cancer diagnosis accuracy through histological findings. We did not assess exposure to drugs known as ITP inducers, because of the impossibility to assess causality. However, drug-induced thrombocytopenia is a rare cause of ITP.8 Moreover, drug-induced thrombocytopenia generally does not become persistent or chronic.52 Therefore, we might have slightly underestimated the incidence of acute, but not that of chronic or persistent, ITP. Bleeding symptoms might have been omitted in hospital stays coding for some patients. That’s why we restricted the study to severe bleedings necessitating active and costly treatments, which would therefore be mentioned. Last, as previously underscored, our study population was patients needing health care. These results cannot be generalized to the overall ITP population including untreated and never hospitalized patients, who are generally asymptomatic and/or have mild thrombocytopenia. Similarly, compared with the entire ITP population, the percentage of cases leading to persistency or chronicity and the frequency of severe bleeding at disease onset might be overestimated.
In conclusion, ITP requiring health care is a rare disease with an overall incidence of ∼3/100 000 person-years in France, increasing up to 9/100 000 person-years in males >75 years of age and peaking in winter. Geographical variations and a north-south increasing gradient were seen in France. Diagnosis of malignancy before or within 6 months after ITP diagnosis is frequent (11%). Confusion or association with myelodysplastic syndromes underlines the need for bone marrow aspiration at diagnosis in older patients.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
The authors thank Laurent Duchet and the Caisse Nationale de l’Assurance maladie des Travailleurs Salariés engineers who performed raw data extraction from the SNIIR-AM database.
Authorship
Contributions: G.M., M.L.-M., and L.S. designed the study and wrote the paper; G.M. and A.P. carried out the data management and statistical analyses; J.-L.M. and B.G. critically reviewed the manuscript; and all authors reviewed the manuscript and gave final approval.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Guillaume Moulis, INSERM UMR 1027, Pharmacoepidemiology Unit (team 6), 37 allées Jules Guesde, 31000 Toulouse, France; e-mail: guillaume.moulis@univ-tlse3.fr.