Key Points
Proinflammatory MKs from mice with GPS drive the extension of myelofibrosis, splenomegaly, and emperipolesis.
The lack of preformed α-granules in Nbeal2−/− platelets leads to protection against cancer metastasis.
Abstract
NBEAL2 encodes a multidomain scaffolding protein with a putative role in granule ontogeny in human platelets. Mutations in NBEAL2 underlie gray platelet syndrome (GPS), a rare inherited bleeding disorder characterized by a lack of α-granules within blood platelets and progressive bone marrow fibrosis. We present here a novel Nbeal2−/− murine model of GPS and demonstrate that the lack of α-granules is due to their loss from platelets/mature megakaryocytes (MKs), and not by initial impaired formation. We show that the lack of Nbeal2 confers a proinflammatory phenotype to the bone marrow MKs, which in combination with the loss of proteins from α-granules drives the development of bone marrow fibrosis. In addition, we demonstrate that α-granule deficiency impairs platelet function beyond their purely hemostatic role and that Nbeal2 deficiency has a protective effect against cancer metastasis.
Introduction
Platelets are anucleate cells that circulate in the blood and their primary role is in hemostasis. They are also essential in processes such as inflammation,1 angiogenesis,2 and wound healing,3 and they contribute to the pathogenesis of myocardial infarction, stroke,4 rheumatoid arthritis,5 and malignant metastasis.6,7 This myriad of actions is explained in part by the unique cargo of their alpha (α) and dense (δ) granules. A vast array of >300 proteins is stored in the α-granules8 including hemostatic and adhesive molecules, such as von Willebrand factor (VWF), fibrinogen, and other coagulation factors, inflammatory peptides such as Interleukin-1 β, transforming growth factor-β, regulated on activation normal T expressed and secreted, and pro-, and anti-angiogenic factors such as vascular endothelial growth factor, platelet-derived growth factor, platelet factor 4 (PF4), and thrombospondin 1 (THBS1).9
Platelets contain 30 to 50 α-granules that account for approximately 10% of their volume.9 The α-granules are formed early during megakaryopoiesis by the fusion of small vesicles from the trans-Golgi network and endocytic vesicles into multivesicular bodies (MVBs)10 then transported through the cytoplasmic extensions of the proplatelet shafts of the mature megakaryocytes (MKs), the bone-marrow residing platelet precursors, where they become trapped in the terminal buds (ie, the nascent platelets).11,12
Gray platelet syndrome (GPS), is a rare autosomal recessive bleeding disorder first reported in 197013 caused by mutations in NBEAL2.14-16 Platelets from GPS patients are large and have a gray appearance caused by an almost complete absence of α-granules. However, some platelets contain MVBs and occasionally platelets with sparse α-granules are seen.17 Other organelles, such as mitochondria, δ-granules, and lysosomes, are unaffected.18 Interestingly, nearly all GPS cases develop fibrosis of the bone marrow in the fourth to fifth decade of life,18 possibly as a consequence of the steady release of cytokines, chemokines, and growth factors normally packaged in the α-granules.
NBEAL2 belongs to a family of genes encoding the beige and Chediak-Higashi syndrome (BEACH)-domain containing proteins.14 Most of these genes encode large multidomain scaffolding proteins with at least 2500 amino acids. Besides NBEAL2 and GPS, mutations in other members of this family are also causative of Mendelian disorders of granule ontogeny and the control of cell volume, with mutations in LYST causing abnormal lysosomal granules and large neutrophils in Chediak-Higashi Syndrome,19 and a mutation in NBEA causing a defect of platelet δ-granules in a case with extreme autism.20
To study the role of Nbeal2 in α-granule formation and GPS-related pathologies, such as myelofibrosis, we generated Nbeal2 null (Nbeal2−/−) mice. We demonstrate that this murine model recapitulates the typical platelet phenotype observed in GPS patients including splenomegaly and myelofibrosis. Analysis of the MKs unexpectedly showed that α-granules are generated, but not retained within MKs. We also show a strong proinflammatory transcriptome signature in MKs from Nbeal2−/− mice, and interestingly platelets formed by these MKs lack the ability to support metastasis of melanoma cancer cells to the lung. In conclusion, we demonstrate for the first time that deficiency in Nbeal2 and a lack of α-granules in GPS platelets has consequences beyond their primary role in hemostasis.
Materials and methods
Generation of Nbeal2−/− mice
Gene targeting for Nbeal2 was performed as part of an international consortium (www.mousephenotype.org) as previously reported,21-23 and described in the supplemental Methods on the Blood Web site and illustrated in supplemental Figure 1A-B.
Adult animals ranging from 4 to 12 months of age were used, including age-matched controls of the same genetic background. Mice were kept in specific pathogen-free conditions, and all procedures were performed according to the United Kingdom Home Office regulations. A minimum of 5 different mice per experiment was used unless otherwise stated.
Platelets and MK isolation and analysis
Blood was withdrawn from the inferior vena cava in either EDTA (5 mM final) or anticoagulant citrate dextrose (111 mM glucose, 71 mM citric acid, 116 mM sodium citrate) as anticoagulants depending on the experiment. Full blood counts were obtained using a Scil Vetabc instrument (Montpellier, France) and blood smears were stained with a May-Grünwald Giemsa (Romanovsky) stain.
Platelet preparation, culture of bone marrow–derived MKs, proplatelet assays, flow cytometry analysis, and generation of cell lysates for western blot analysis have been described before and are detailed elsewhere24 and in the supplemental Methods.
Transmission electron microscopy of platelets, bone marrow MKs, and in vitro cultured MKs
Fixed solutions of platelets, bone marrow–derived MKs, and cultured MKs were rinsed in 0.1 M N-2-hydroxyethylpiperazine-N′-2-ethanesulfonic acid buffer 5 times and processed as described in the supplemental Methods.
Immunostaining of platelets and MKs
Fixed platelets were incubated onto coverslips at 37°C for 90 minutes. Purified cultured MKs were adhered onto fibrinogen-coated slides and then fixed. A detailed description of immunostaining is provided elsewhere25 and in the supplemental Methods.
Analysis of tissue sections
The humerus, femur, tibia, fibula, and spleen were taken and placed in a formalin fixative. After decalcification they were processed as stated in the supplemental Methods.
Expression arrays and qPCR in cultured MKs
RNA was isolated from cultured MKs using the RNeasy kit (Qiagen, Manchester, UK) and complimentary DNA made using Superscript III or SS VILO (first Strand kit; Invitrogen). Quantitative polymerase chain reaction (qPCR) assays were performed on an Mx3005P qPCR System (Agilent Technologies, UK) using primers described in supplemental File 1. Expression levels were assayed using Illumina Mus 6v2 expression chip. Data processing and analysis were performed as described elsewhere.24 Gene set enrichment analysis was carried out using Gene Set Enrichment Analysis.26 Version 4 gene sets of Gene Ontology (GO) molecular function were used with a weighted scoring scheme of the Student t scores.
Experimental metastasis assay
Murine metastatic melanoma B16-F10 cells were cultured in Dulbecco’s modified Eagle medium supplemented with 10% (vol/vol) fetal bovine serum, 2 mM glutamine, and 100 U/mL penicillin/streptomycin. Cells in the log-phase growth were harvested, resuspended in phosphate-buffered saline (PBS), and 5 × 105 cells (in 0.1 mL) were injected into the tail vein of 6- to 8-week-old sex-matched syngeneic control (n = 9) and Nbeal2−/− (n = 5) mice. Additional groups included Nbeal2−/− animals (n = 4) transfused with 240 × 106 platelets previously isolated from control mice, or PBS (n = 3) and control mice injected with PBS (n = 5) 4 hours prior to the injection of the melanoma cells. The mice were sacrificed 10 days later. The lungs were removed, rinsed in PBS, and the number of metastatic foci were counted under a dissecting microscope.
Statistics
Results are shown as the mean ± the standard error of the mean. Statistical analysis was performed using the unpaired Student t test. P < .05 was considered statistically significant.
Results
The Nbeal2−/− mouse has a GPS phenotype
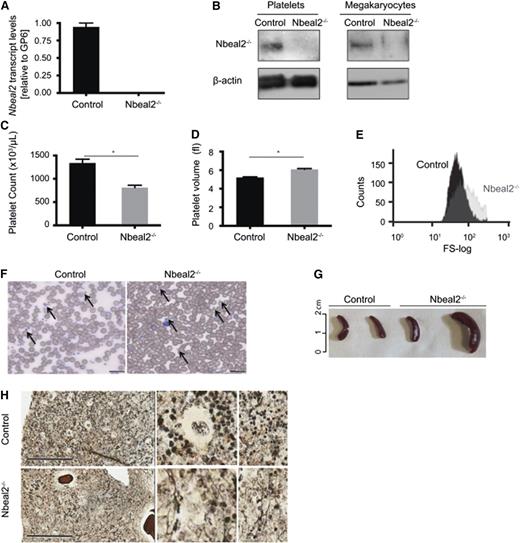
The absence of Nbeal2 at transcript and protein level was confirmed in Nbeal2tm1a Wtsi/tm1a Wtsi (hereafter referred to as Nbeal2−/−) bone marrow–derived MKs and blood platelets (Figure 1A-B). The platelet count was reduced by almost 50% (1377 ± 84 × 103 vs 811 ± 49 × 103 platelets/μL for control and Nbeal2−/− animals, respectively; P = 3.1 × 10−7) (Figure 1C) and the mean platelet volume was increased (5.2 ± 0.1 vs 6.1 ± 0.1 fL; P = 8.1 × 10−8) (Figure 1D), also shown by flow cytometric analysis (Figure 1E), and the “grayish” appearance was confirmed (Figure 1F). Crucially, splenomegaly and bone marrow myelofibrosis, clinical features typical of GPS cases, were observed in Nbeal2−/− animals older than 9 months (Figure 1G-H, respectively).
Deficiency of Nbeal2 in mice leads to GPS. (A) Transcript levels of Nbeal2 by qPCR in RNA isolated from in vitro cultured MKs. Results are normalized to levels of GP6 (n = 3). (B) Protein levels of Nbeal2 in platelets and MKs from Nbeal2−/− and control mice. β-actin included as an internal loading control (n = 3). (C) Platelet count in Nbeal2−/− and control animals (n = 13). (D) Mean platelet volume in Nbeal2−/− and control mice (n = 13). (E) Size distribution of the gated platelet population visualized in a representative flow cytometry histogram (forward scatter). (F) Representative May-Grünwald Giemsa stain of blood smears. Arrows indicate the presence of platelets. Platelets are larger and appear gray in Nbeal2−/− samples. Bars represent 20 μm. (G) Different degrees of splenomegaly were observed in Nbeal2−/− mice beyond the age of 9 months. Age-matched control mice were used for comparison (n = 4). (H) A representative reticulin staining of bone sections from mice at 9 months of age showing myelofibrosis in Nbeal2−/− marrow. Bars represent 200 μm. *P < .05.
Deficiency of Nbeal2 in mice leads to GPS. (A) Transcript levels of Nbeal2 by qPCR in RNA isolated from in vitro cultured MKs. Results are normalized to levels of GP6 (n = 3). (B) Protein levels of Nbeal2 in platelets and MKs from Nbeal2−/− and control mice. β-actin included as an internal loading control (n = 3). (C) Platelet count in Nbeal2−/− and control animals (n = 13). (D) Mean platelet volume in Nbeal2−/− and control mice (n = 13). (E) Size distribution of the gated platelet population visualized in a representative flow cytometry histogram (forward scatter). (F) Representative May-Grünwald Giemsa stain of blood smears. Arrows indicate the presence of platelets. Platelets are larger and appear gray in Nbeal2−/− samples. Bars represent 20 μm. (G) Different degrees of splenomegaly were observed in Nbeal2−/− mice beyond the age of 9 months. Age-matched control mice were used for comparison (n = 4). (H) A representative reticulin staining of bone sections from mice at 9 months of age showing myelofibrosis in Nbeal2−/− marrow. Bars represent 200 μm. *P < .05.
Nbeal2−/− platelets lack α-granules
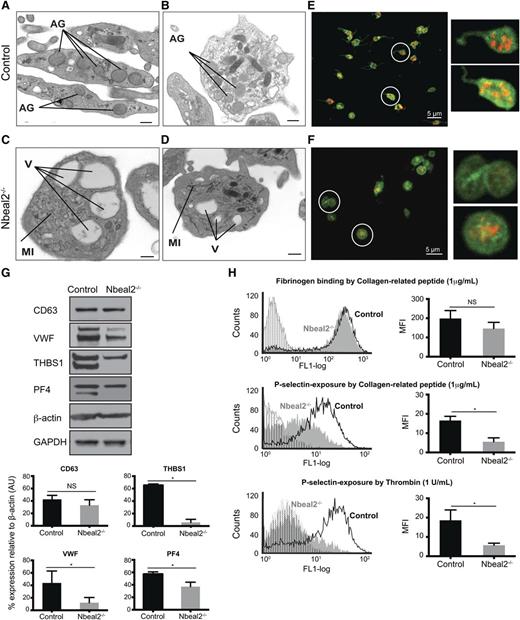
Platelet ultrastructure was studied by transmission electron microscopy (TEM). Control platelets had the typical discoid shape and contained numerous α-granules (Figure 2A-B). As expected, platelets from Nbeal2−/− animals were larger and rounder in shape with some platelets showing abnormal membrane formations with linear or tubular sheet inclusions (Figure 2C-D; supplemental Figure 2). As in human GPS platelets, there was a reduction in α-granule contents. The typical vacuole-like organelles were present, with some containing electrodense material (Figure 2C-D). The TEM results were confirmed by immunofluorescence staining for α-granule proteins (such as VWF) by confocal microscopy. VWF was shown to have the typical granular distribution in control platelets (Figure 2E), whereas Nbeal2−/− platelets either completely lacked VWF (Figure 2F) or displayed a diffuse rather than granular distribution. Total platelet protein content was analyzed by western blot analysis using platelet lysates. Although CD63 (LAMP-3), a marker of δ-granules and lysosomes, was present at similar levels in control and Nbeal2−/− platelets, there was a significant reduction in α-granule proteins, such as VWF, THBS1, and PF4 in the latter (Figure 2G).
Impaired platelet function in Nbeal2−/− mice. (A-B) Ultrastructure of platelets from control mice showing abundant α-granules (AG), among other organelles. (C-D) Ultrastructure of Nbeal2−/− platelets showing a significant reduction of α-granules, several vacuoles (V) and membrane inclusions (MI). (E-F) Immunolabeling of VWF (red) and CD41 (green) in control (E) and Nbeal2−/− (F) platelets. Images correspond to the maximum intensity projections of an image stack across an entire platelet section. Insets: A higher magnification of the circled platelets is shown for clarification. VWF is restricted to the α-granules in control platelets, whereas its distribution is more diffuse in Nbeal2−/− platelets. Note also the absence of VWF in some Nbeal2−/− platelets. (G) Representative immunoblots of α-granule proteins VWF, THBS1 and PF4 in control and Nbeal2−/− platelet lysates. CD63 (LAMP-3), a lysosomal/dense granule marker, and β-actin and glyceraldehyde-3-phosphate dehydrogenase included as loading controls. Bottom graphs show the densitometry analysis performed using ImageJ (n = 3). *P < .05. (H) Flow cytometry analysis of platelet activation induced by 1 μg/mL collagen-related peptide and 1 U/mL thrombin (n = 4) showing equivalent fibrinogen binding to Nbeal2−/− and control platelets (top panel, only shown for collagen-related peptide) in contrast reduced P-selectin exposure in Nbeal2−/− platelets with both agonists (middle and bottom panels). Plots with vertical lines correspond to basal platelet activation in the absence of agonist. For all graphs, bars represent mean ± standard error of the mean. Scale bars represent 100 nm (A, C) and 500 nm (B, D). *P < .05. NS, nonsignificant; MFI, mean fluorescent intensity.
Impaired platelet function in Nbeal2−/− mice. (A-B) Ultrastructure of platelets from control mice showing abundant α-granules (AG), among other organelles. (C-D) Ultrastructure of Nbeal2−/− platelets showing a significant reduction of α-granules, several vacuoles (V) and membrane inclusions (MI). (E-F) Immunolabeling of VWF (red) and CD41 (green) in control (E) and Nbeal2−/− (F) platelets. Images correspond to the maximum intensity projections of an image stack across an entire platelet section. Insets: A higher magnification of the circled platelets is shown for clarification. VWF is restricted to the α-granules in control platelets, whereas its distribution is more diffuse in Nbeal2−/− platelets. Note also the absence of VWF in some Nbeal2−/− platelets. (G) Representative immunoblots of α-granule proteins VWF, THBS1 and PF4 in control and Nbeal2−/− platelet lysates. CD63 (LAMP-3), a lysosomal/dense granule marker, and β-actin and glyceraldehyde-3-phosphate dehydrogenase included as loading controls. Bottom graphs show the densitometry analysis performed using ImageJ (n = 3). *P < .05. (H) Flow cytometry analysis of platelet activation induced by 1 μg/mL collagen-related peptide and 1 U/mL thrombin (n = 4) showing equivalent fibrinogen binding to Nbeal2−/− and control platelets (top panel, only shown for collagen-related peptide) in contrast reduced P-selectin exposure in Nbeal2−/− platelets with both agonists (middle and bottom panels). Plots with vertical lines correspond to basal platelet activation in the absence of agonist. For all graphs, bars represent mean ± standard error of the mean. Scale bars represent 100 nm (A, C) and 500 nm (B, D). *P < .05. NS, nonsignificant; MFI, mean fluorescent intensity.
We went on to characterize platelet function. First, we carried out flow cytometry analysis to assess the expression of the main platelet receptors on the surface of resting platelets, which showed no differences between groups of control and Nbeal2−/− mice for integrin αIIbβ3 (CD41/61), integrin α2 (CD49b), glycoprotein (GP) V (CD42d), and GPVI (supplemental Figure 3). Engaging platelets with cross-linked collagen-related peptide (CRP) and thrombin showed equivalent levels of activation between groups of animals as determined by the level of fibrinogen binding to αIIbβ3 (Figure 2H top panel), consistent with preservation of the major platelet activation pathways. In contrast, exposure of the α-granule membrane protein P-selectin on the outer surface of platelets after their activation with CRP and thrombin was significantly reduced in Nbeal2−/− mice (Figure 2H middle and bottom panels), consistent with the reduction in α-granules.
Nbeal2−/− MKs have a maturation defect, but proplatelet formation is maintained
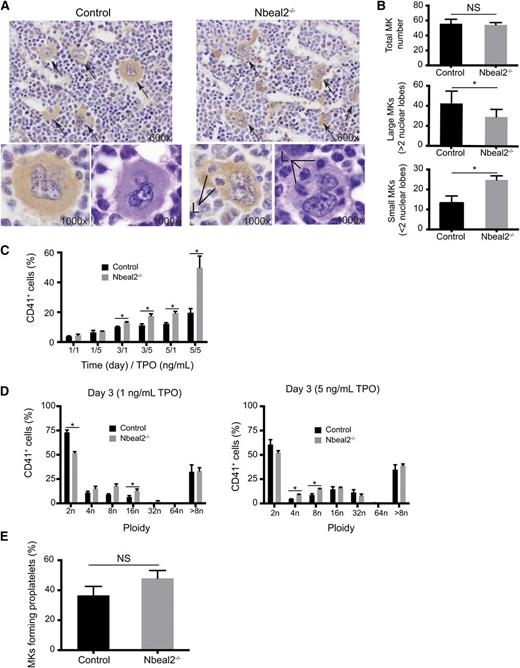
Bone marrow sections from Nbeal2−/− and control mice (n = 5) were stained with hematoxylin and eosin and an immunoperoxidase method for the platelet glycoprotein αIIbβ3 complex (CD41). The number and morphology of the MKs in the bone marrow sections were assessed by a hematopathologist who was blinded to the Nbeal2 status of the mice (Figure 3A). The total number of MKs was comparable in Nbeal2−/− and control mice (Figure 3B, top panel). However, Nbeal2−/− mice had significantly fewer large MK with multilobated nuclei (Figure 3B, middle panel; P = .038) and an increase in small MKs with reduced nuclear lobation compared with control mice (Figure 3B, bottom panel; P = .00023). Furthermore, the MKs in Nbeal2−/− mice had prominent emperipolesis of neutrophils (Figure 3A); this was not seen in MKs of the control samples. These distinctive MK morphologic features enabled all Nbeal2−/− and control mice to be identified with 100% accuracy. To further study the potential effects of emperipolesis on MKs, including cell death, immunolabeling of Bcl-XL and cleaved caspase 3 was carried out in bone marrow sections. No difference was observed for either staining between control and Nbeal2−/− MKs (supplemental Figure 4).
Abnormal MK maturation in Nbeal2−/− mice. (A) CD41 labeling and hematoxylin and eosin staining of bone sections (×60) in control and Nbeal2−/− mice. Arrows point at MKs. Two representative large polyploid MKs are shown in the bottom panels at high magnification (×100). Note the presence of leukocytes (L) within MKs (emperipolesis) at high magnification (×100) in the Nbeal2−/− samples. (B) Quantitation of MKs in bone sections from (A) (5 different fields in 5 mice). Similar total MK numbers in control and Nbeal2−/− mice (top panel), but the numbers of large MKs with more than 2 nuclear lobes (middle panel) and the numbers of small MKs with 2 or less nuclear lobes (bottom panel) are significantly different in Nbeal2−/− mice. (C) Percentage of CD41+ cells in bone marrow cultures in the presence of thrombopoietin (TPO) (1 and 5 ng/mL) at different time points (1, 3, and 5 days) (n = 4). (D) Ploidy profile of CD41+ cells at day 3 (n = 4). (E) Percentage of proplatelet-forming MKs upon adhesion to fibrinogen of cultured MKs purified by bovine serum albumin gradient (n = 4). For all graphs, bars represent mean ± standard error of the mean. *P < .05. NS, nonsignificant.
Abnormal MK maturation in Nbeal2−/− mice. (A) CD41 labeling and hematoxylin and eosin staining of bone sections (×60) in control and Nbeal2−/− mice. Arrows point at MKs. Two representative large polyploid MKs are shown in the bottom panels at high magnification (×100). Note the presence of leukocytes (L) within MKs (emperipolesis) at high magnification (×100) in the Nbeal2−/− samples. (B) Quantitation of MKs in bone sections from (A) (5 different fields in 5 mice). Similar total MK numbers in control and Nbeal2−/− mice (top panel), but the numbers of large MKs with more than 2 nuclear lobes (middle panel) and the numbers of small MKs with 2 or less nuclear lobes (bottom panel) are significantly different in Nbeal2−/− mice. (C) Percentage of CD41+ cells in bone marrow cultures in the presence of thrombopoietin (TPO) (1 and 5 ng/mL) at different time points (1, 3, and 5 days) (n = 4). (D) Ploidy profile of CD41+ cells at day 3 (n = 4). (E) Percentage of proplatelet-forming MKs upon adhesion to fibrinogen of cultured MKs purified by bovine serum albumin gradient (n = 4). For all graphs, bars represent mean ± standard error of the mean. *P < .05. NS, nonsignificant.
Then we analyzed in vitro MK differentiation and maturation from bone marrow cultures using two submaximal concentrations of thrombopoietin (1 and 5 ng/mL) at 2 time points (3 and 5 days). The number of megakaryocytic CD41+ cells was similar in control and Nbeal2−/− samples at day 1. However, the difference in the percentage of CD41+ cells between control and Nbeal2−/− samples gradually increased up to 2.5-fold at day 5 with 5 ng/mL thrombopoietin (Figure 3C). In contrast, the proportion of high ploidy MKs (cells with 8N and above) within the CD41+ population did not differ between the cultures (Figure 3D).
Proplatelet formation was analyzed by adhering bone marrow–derived cultured MKs onto fibrinogen after selection of the most mature MKs (≥8N) over a bovine serum albumin gradient. The rate of proplatelet formation was similar in control and Nbeal2−/− cells (Figure 3E).
Lack of Nbeal2 does not affect α-granule formation, packaging, or transport to the budding proplatelets
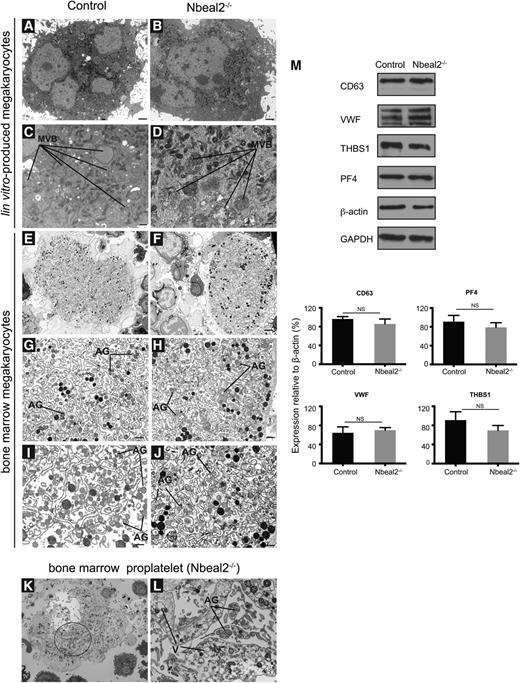
TEM assessment of bone marrow culture–derived MKs showed no major differences between Nbeal2−/− cells and controls (Figure 4A-B). In particular, there were equal numbers of MVBs, the precursors of α-granules, in both sample sets (Figure 4C-D). As only a few truly mature α-granules were seen in the cultured MKs, we went on to study MKs directly flushed from the bone marrow. Control MKs showed a well-defined demarcation membrane system with the presence of “platelet territories” and mature α-granules (Figure 4E, G, I). Nbeal2−/− MKs appeared to have a more rudimentary demarcation membrane system and a minor degree of vacuolization but did not show any significant abnormalities, such as membrane inclusions (Figure 4F,H,J-L). Strikingly, mature α-granules were present in Nbeal2−/− MKs and were of similar size and morphology to those in control MKs, although their density and opacity was slightly decreased (Figure 4G-J). The striking emperipolesis documented by light microscopy on bone marrow sections was also observed in Nbeal2−/− MKs by TEM (supplemental Figure 5). The presence of α-granules in both control and Nbeal2−/− MKs was confirmed by immunohistochemistry. Staining from VWF showed a similar granular distribution in both sets of samples (supplemental Figure 6). In keeping with these observations, western blot analysis of Nbeal2−/− and control-cultured MK lysates confirmed similar cellular content not only for CD63, but also for α-granule proteins, such as VWF, THBS1, and PF4 (Figure 4M).
Ultrastructure and protein analysis of MKs. (A-D) Ultrastructure of culture-derived MKs from control (A) and Nbeal2−/− (B) mice at low magnification (A-B) and high magnification (C-D), respectively. MVB precursors of α-granules are present in both control and Nbeal2−/− MKs. (E-F) Ultrastructure of bone marrow MKs in control and Nbeal2−/− animals at low magnification. (G-J) Ultrastructure of bone marrow MKs from control and Nbeal2−/− mice at high magnification. A well-defined demarcation membrane system with the presence of platelet territories and α-granules (AG) in control cells (G, I). The demarcation membrane system in Nbeal2−/− MKs is not well-defined (H,J), however, α-granules (AG) are present. (K) Low magnification of a proplatelet-forming MK from Nbeal2−/− bone marrow. (L) High magnification of the circled area in (K) highlights the presence of AG inside the emerging platelet fields, and vacuoles (V) within the MK cytoplasm. (M) Representative immunoblots of α-granule proteins (VWF) (THBS1 and PF4) in control and Nbeal2−/− MKs. CD63 (LAMP-3), a lysosomal/dense granule marker, and β-actin and glyceraldehyde-3-phosphate dehydrogenase included as loading controls. Below, graphs show the densitometry analysis performed using ImageJ (n = 3). Bars represent mean ± standard error of the mean. NS, nonsignificant. Bar represents 2 μm (A-B,E-F,K) and 500 nm (C-D,G-J,L). Representative images were selected from 5 different mice per group.
Ultrastructure and protein analysis of MKs. (A-D) Ultrastructure of culture-derived MKs from control (A) and Nbeal2−/− (B) mice at low magnification (A-B) and high magnification (C-D), respectively. MVB precursors of α-granules are present in both control and Nbeal2−/− MKs. (E-F) Ultrastructure of bone marrow MKs in control and Nbeal2−/− animals at low magnification. (G-J) Ultrastructure of bone marrow MKs from control and Nbeal2−/− mice at high magnification. A well-defined demarcation membrane system with the presence of platelet territories and α-granules (AG) in control cells (G, I). The demarcation membrane system in Nbeal2−/− MKs is not well-defined (H,J), however, α-granules (AG) are present. (K) Low magnification of a proplatelet-forming MK from Nbeal2−/− bone marrow. (L) High magnification of the circled area in (K) highlights the presence of AG inside the emerging platelet fields, and vacuoles (V) within the MK cytoplasm. (M) Representative immunoblots of α-granule proteins (VWF) (THBS1 and PF4) in control and Nbeal2−/− MKs. CD63 (LAMP-3), a lysosomal/dense granule marker, and β-actin and glyceraldehyde-3-phosphate dehydrogenase included as loading controls. Below, graphs show the densitometry analysis performed using ImageJ (n = 3). Bars represent mean ± standard error of the mean. NS, nonsignificant. Bar represents 2 μm (A-B,E-F,K) and 500 nm (C-D,G-J,L). Representative images were selected from 5 different mice per group.
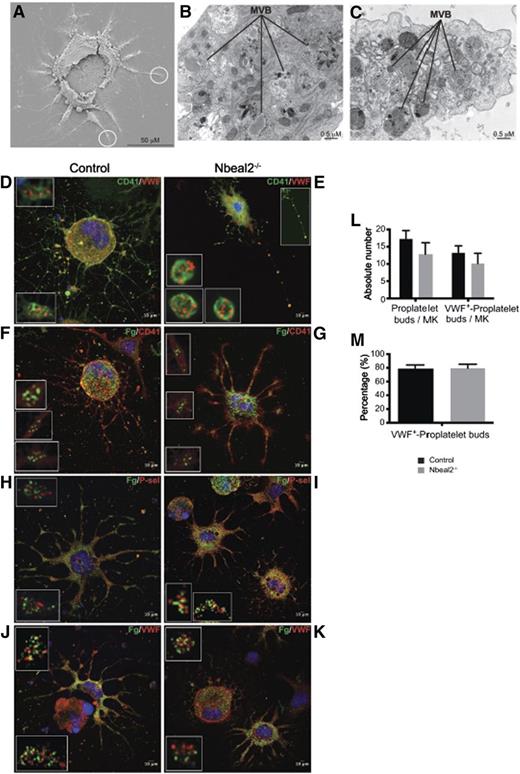
The apparent discrepancy of these results between the Nbeal2−/− platelets and MKs led us to assess whether the lack of Nbeal2 affects transport and accumulation of granules in the proplatelet buds. To this end, we carried out proplatelet assays using mature culture-derived MKs as described above and visualized the proplatelet buds by scanning electron microscopy, TEM, and confocal microscopy (Figure 5A-K). This confirmed the presence of α-granules at different levels of maturation, including MVBs within the cytoplasm of the proplatelet territories in both control and Nbeal2−/− samples (Figure 5A-C). Immunolabeling for VWF showed the typical granular distribution previously documented, but in particular, comparable accumulation of α-granules in the proplatelet buds in both sets of samples (Figure 5D-E). The α-granule content is the result of both intracellular protein synthesis and endocytosis of extracellular proteins, such as fibrinogen. To assess whether the lack of Nbeal2 potentially affects the latter cellular process, MKs were incubated with fluorescein isothiocyanate-labeled fibrinogen for 24 hours during culture prior to performing the proplatelet assay. Fluorescein isothiocyanate-fibrinogen showed a similar granular distribution to that seen for VWF, not only in the core of the cells, but also in the proplatelet buds (Figure 5F-G). Coimmunolabeling with other α-granule proteins of endogenous origin, namely P-selectin (Figure 5H-I) and VWF (Figure 5J-K) confirmed that both endogenous and endocytosed proteins accumulate in granules within the terminal buds of both control and Nbeal2−/− proplatelets, although segregating in distinct population of granules, a phenomenon that has been previously described.27 Additional deconvolved images of the raw confocal stacks were also generated and are available for viewing (supplemental Figure 7-8). The number of proplatelets and VWF+ proplatelet buds per cell were quantified and revealed no statistically significant differences (Figure 5L-M).
Analysis of α-granules and their content in proplatelet-forming MKs by electron and confocal microscopy. (A) Low magnification of a representative control proplatelet-forming MK by scanning electron microscopy showing proplatelet-like territories emerging from the main core of the cell. (B-C). Higher magnification of the circled areas in (A) seen by TEM. The developing proplatelets contain MVBs, precursors of α-granules. (D-E) Immunolabeling of CD41 and VWF in proplatelet-forming MKs by confocal microscopy in control (D) and Nbeal2−/− (E) cells. VWF is restricted to the MVBs bodies observed in (B-C). The inset at the top-right corner in (E) highlights the presence of a thin MK filament bridging several emerging platelet-like particles. (F-G) Immunolabeling of CD41 in proplatelet-forming MKs previously cultured in the presence of fluorescein isothiocyanate (FITC)-fibrinogen. Fibrinogen is endocytosed and packaged into α-granules in control (F) and Nbeal2−/− (G) MKs, and transported through the proplatelet shafts. (H-I) Proplatelet-forming MKs cultured with FITC fibrinogen, as stated in (F), and P-selectin-immunolabeled in control (H) and Nbeal2−/− (I) samples. (J-K) Proplatelet-forming MKs cultured in the presence of FITC-fibrinogen, as stated in (F), and VWF-immunolabeled in control (J) and Nbeal2−/− (K) samples. (L) Quantitation of proplatelets bud in MK culture. (M) Percentage of proplatelets buds containing the α-granule protein VWF. (D-K) Insets represent a higher magnification of platelet-like particles showing a similar granular distribution of P-selectin, VWF, and fibronogen in control and Nbeal2−/− cells. (D-K) The nucleus was stained with 4,6 diamidino-2-phenylindole (blue). Representative images were selected from 5 different mice per group.
Analysis of α-granules and their content in proplatelet-forming MKs by electron and confocal microscopy. (A) Low magnification of a representative control proplatelet-forming MK by scanning electron microscopy showing proplatelet-like territories emerging from the main core of the cell. (B-C). Higher magnification of the circled areas in (A) seen by TEM. The developing proplatelets contain MVBs, precursors of α-granules. (D-E) Immunolabeling of CD41 and VWF in proplatelet-forming MKs by confocal microscopy in control (D) and Nbeal2−/− (E) cells. VWF is restricted to the MVBs bodies observed in (B-C). The inset at the top-right corner in (E) highlights the presence of a thin MK filament bridging several emerging platelet-like particles. (F-G) Immunolabeling of CD41 in proplatelet-forming MKs previously cultured in the presence of fluorescein isothiocyanate (FITC)-fibrinogen. Fibrinogen is endocytosed and packaged into α-granules in control (F) and Nbeal2−/− (G) MKs, and transported through the proplatelet shafts. (H-I) Proplatelet-forming MKs cultured with FITC fibrinogen, as stated in (F), and P-selectin-immunolabeled in control (H) and Nbeal2−/− (I) samples. (J-K) Proplatelet-forming MKs cultured in the presence of FITC-fibrinogen, as stated in (F), and VWF-immunolabeled in control (J) and Nbeal2−/− (K) samples. (L) Quantitation of proplatelets bud in MK culture. (M) Percentage of proplatelets buds containing the α-granule protein VWF. (D-K) Insets represent a higher magnification of platelet-like particles showing a similar granular distribution of P-selectin, VWF, and fibronogen in control and Nbeal2−/− cells. (D-K) The nucleus was stained with 4,6 diamidino-2-phenylindole (blue). Representative images were selected from 5 different mice per group.
Nbeal2−/− MKs have a proinflammatory profile
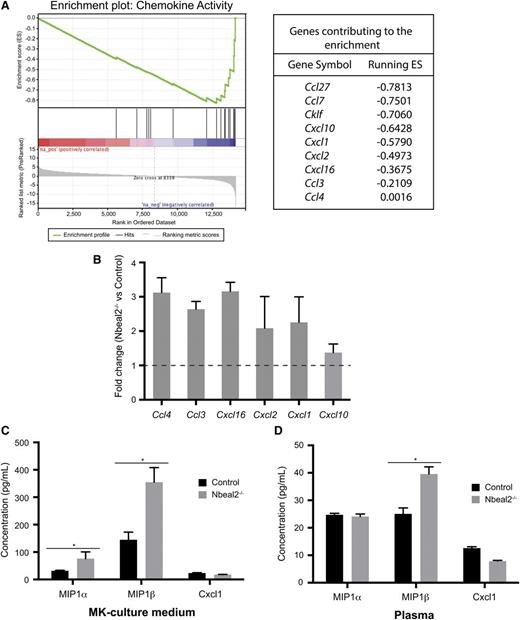
Both GPS mice and patients develop premature myelofibrosis of the bone marrow with aging, and it may be that this is caused by the proinflammatory nature of GPS MKs and platelets. To provide insights into the role of Nbeal2 in MKs, we analyzed the RNA from culture-derived MKs on gene expression arrays. Analysis showed that 157 genes were differentially expressed with a false discovery rate <0.1. The 25 most differentially expressed transcripts (overexpressed and underexpressed in Nbeal2−/−) are listed in supplemental File 2. Absence of Nbeal2 was confirmed at the transcript level. Term enrichment analysis based on GO molecular function identified 20 gene sets significantly enriched in Nbeal2−/− relative to control MKs (false discovery rate < 0.25) (supplemental File 3). The most significant GO term enrichment concerned genes encoding chemokines (Figure 6A). A set of chemokine transcripts (namely Ccl3, Ccl4, Cxcl16, Cxcl1, Cxcl2, and Cxcl10) was selected for confirmation testing of the observed transcript level differences by qPCR and were all replicated (Figure 6B). Ccl3 and Ccl4 encode macrophage inflammatory protein (MIP) 1α and 1β, respectively, well-known proinflammatory chemokines increased in primary myelofibrosis,28,29 whereas Cxcl1 is a neutrophil chemoattractant.30 To determine whether the increased transcript levels were mirrored at the protein level, the levels of MIP1α, MIP1β, and Cxcl1 were determined by enzyme-linked immunosorbent assay in MK culture supernatant and plasma. MIP-1α and MIP-1β levels were higher in the culture supernatant of Nbeal2−/− MKs (Figure 6C), and MIP-1β was significantly increased (P = .0019) in plasma of Nbeal2−/− animals at 1 year of age when compared with age-matched controls (Figure 6D).
Gene expression array in culture-derived MKs. (A) Enrichment of the “chemokine activity” gene set in Nbeal2−/− MKs with the genes contributing to this enrichment. (B) Fold change of the 6 most significant genes identified in (A) in Nbeal2−/− vs control MKs generated by qPCR using different samples as those used in the array (n = 3). (C) Plasma levels of the chemokines MIP-1α (Ccl3), MIP-1β (Ccl4), and Cxcl1 in MK culture supernatant. (D) Levels of these chemokines in plasma of control and Nbeal2−/− mice (n = 5). Bars represent mean ± standard error of the mean. *P < .05.
Gene expression array in culture-derived MKs. (A) Enrichment of the “chemokine activity” gene set in Nbeal2−/− MKs with the genes contributing to this enrichment. (B) Fold change of the 6 most significant genes identified in (A) in Nbeal2−/− vs control MKs generated by qPCR using different samples as those used in the array (n = 3). (C) Plasma levels of the chemokines MIP-1α (Ccl3), MIP-1β (Ccl4), and Cxcl1 in MK culture supernatant. (D) Levels of these chemokines in plasma of control and Nbeal2−/− mice (n = 5). Bars represent mean ± standard error of the mean. *P < .05.
The α-granule deficiency confers protection against cancer metastasis
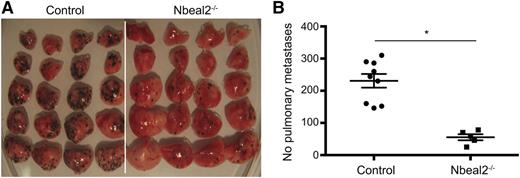
Platelet function extends beyond hemostasis; therefore, we went on to analyze the potential relevance of platelet α-granules in tumorigenesis, and in metastatic spread in particular. For this we used an in vivo model of experimental lung metastasis. Ten days after intravenous injection of murine melanoma B16-F10 cells, lung metastases were quantified. Strikingly, we found a marked reduction, by almost 80%, in the number of pulmonary metastases in Nbeal2−/− mice (231 ± 21 vs 56 ± 21; P = .001) (Figure 7). To confirm the key role of platelets and particularly the role of α-granules in metastasis, platelets isolated from control mice were transfused into Nbeal2−/− animals prior to injecting the melanoma cells. The platelet count incremented by 119 ± 20 × 109/L (P < .05) compared with mice injected with PBS alone and was maintained over the first 48 hours posttransfusion. One of the mice transfused with PBS had to be culled at day 6 and showed no lung metastases. At day10, PBS control mice had 115 ± 18 pulmonary metastases, whereas platelet-transfused and PBS-injected Nbeal2−/− animals had 95 ± 7 and 8 ± 3, respectively (P = .004).
Reduced metastasis in Nbeal2−/− mice. Lung metastasis was induced by tail vein injection of 5 × 105 mouse melanoma B16-F10 cells. Lungs were taken after 10 days and the number of pulmonary metastases were counted on all 5 lobes of the lung. Representative lung metastases in control (n = 9) and Nbeal2−/− mice (n = 5) (A) and quantitation (B). Bar represents mean ± standard error of the mean. *P = .0001.
Reduced metastasis in Nbeal2−/− mice. Lung metastasis was induced by tail vein injection of 5 × 105 mouse melanoma B16-F10 cells. Lungs were taken after 10 days and the number of pulmonary metastases were counted on all 5 lobes of the lung. Representative lung metastases in control (n = 9) and Nbeal2−/− mice (n = 5) (A) and quantitation (B). Bar represents mean ± standard error of the mean. *P = .0001.
Discussion
For more than 40 years, cellular and biological studies of GPS have performed using human samples from clinical cases and therefore, most of the reported data relates to the platelet phenotype due to challenges in isolating abundant MKs from human blood samples. In 2011, 3 groups reported NBEAL2 as the gene underlying GPS, and consequently, 2 reports have been recently published describing an Nbeal2−/− mouse model that recapitulates the platelet phenotype observed in human GPS cases.31,32 Although our study uses a novel Nbeal2−/− mouse with a different genetic strategy, the platelet hallmarks of GPS are recapitulated. In addition, we present data that provides a role for Nbeal2 in platelets beyond their classical hemostatic role. We demonstrate that the lack of α-granules in platelets is the result of an impaired retention rather than a lack of granule generation. In addition, we show that Nbeal2 deficiency in MKs leads to distinctive pathological findings such as myelofibrosis and a proinflammatory phenotype.
In agreement with previous studies,31,32 the hemostatic platelet function of Nbeal2−/− mice was shown to be similarly impaired. In one study, this was shown to correlate with a decrease in the thrombo-inflammatory process of hypoxic brain damage and impaired wound healing, suggesting an effect of platelet α-granule deficiency beyond primary hemostasis. In contrast with the previous mouse models, we observed the development of bone marrow fibrosis and splenomegaly (a feature of human GPS) in older animals. It is well documented that, in the context of myeloproliferative neoplasms, proinflammatory cytokines play a central role in clinical symptoms and, probably, pathogenicity of the disease.29 In the context of JAK2V617F-positive myelofibrosis, one of the main benefits of treating patients with JAK2 inhibitors has been the control of inflammation and the raised cytokine levels leading to a resolution of systemic symptoms.33 Transcriptome analysis of cultured MKs followed by qPCR validation clearly showed that Nbeal2−/− MKs overexpress transcripts for several chemokines, confirming a link between Nbeal2 deficiency and inflammation in the context of GPS. Significantly, we found that plasma levels of at least one of those chemokines (MIP-1β) were raised, suggesting a potential systemic effect beyond the bone marrow. Emperipolesis of neutrophils was a prominent feature of the Nbeal2−/− marrow, which may be explained through the paracrine effect of chemoattractants, such as Cxcl1 released by Nbeal2−/− MKs. There is evidence in myeloproliferative neoplasms that emperipolesis can be driven by an increased level of P-selectin exposure on the surface of MKs, which we did not observe in Nbeal2−/− MKs, and that emperipolesis may induce MK death leading to the release of granule content and subsequent fibrosis.34 However, immunolabeling of cleaved caspase-9 and Bcl-XL in bone marrow showed no differences between mice, suggesting that this may not be the case in the context of GPS. Although we cannot rule out that immune cells within the bone marrow participate in the inflammatory phenotype, overproduction of chemokines by MKs in liquid cultures depleted of inflammatory cells suggest that the MKs are one of the primary drivers of inflammation in GPS.
Myelofibrosis in GPS patients may be the result of spontaneous release of α-granule proteins from the MKs.18 One of our most striking observations was the contrast between the circulating platelets, which were almost entirely devoid of α-granules, and the MKs where granule content was preserved. Analysis of the MKs by electron microscopy and immunofluorescence showed normal production/maturation of MVBs and mature α-granules, preserved packaging of both endogenous and endocytosed granule proteins, and normal transport of the α-granules to the proplatelet tips. This indicates that the lack of Nbeal2 does not hamper granule synthesis, but suggests lack of retention of α-granules within the cell. It is unclear whether this loss occurs in platelets after their release into the circulation and/or at the level of the MKs within the bone marrow. If the latter, the loss can be compensated by de novo synthesis explaining the similar granule content in control and Nbeal2−/− MKs. We measured plasma concentration of granule proteins, such as VWF and fibrinogen, and we found no difference between Nbeal2−/− and control samples (data not shown), which was also reported in the previous studies,31,32 but this does not exclude a paracrine effect of the “leaking” granule proteins within the bone marrow potentially contributing to the proinflammatory effects of the MKs mentioned above and the development of myelofibrosis.
The observation of α-granules within the Nbeal2−/− MKs is in contrast with the previously published mouse studies, which used a different knockdown strategy to our own. We can, however, draw a parallel to the few reported studies of MKs isolated from humans with GPS: some studies report the absence of α-granules in the MKs,35,36 whereas others showed the presence of abnormal α-granules in the MKs by immunolabeling37 further confirmed by TEM.38 Indeed, supplemental Figure 9 illustrates MKs from a patient with GPS (carrying the mutation L388P in NBEAL2) that does contain granules, just as it is the case for the mouse MKs reported here. One possible explanation for these differences would be sample preparation and the level of maturity of the MKs analyzed in particular (culture-derived vs extracted from bone marrow and immature cells vs proplatelet forming MKs). We cannot rule out that these differences are explained by the variation in genotype between patients. Mutations in human NBEAL2 have been found to affect different domains of the NBEAL2 protein, which may lead to a spectrum of phenotypes within the MKs, whereas the end product, the platelets, is universally depleted of α-granules. A better understanding of the hitherto unknown function of the NBEAL2 protein and of its partners will be crucial in shedding light on how its deficiency/dysfunction affects α-granule formation and retention within the cell.
The clear demonstration that a lack of platelet α-granules has a link with a nonhemostatic platelet function led us to investigate whether the same was true for the role of platelets in malignancies. To this end, we used an in vivo model of cancer metastasis. Although this model addresses chiefly homing and invasion of malignant cells into tissue in the somewhat artificial context of large numbers of cells directly injected into the bloodstream, the striking data place the platelet, and its α-granules, in particular, at the center of the metastatic process. The rate of metastasis was markedly decreased in Nbeal2−/− animals, although transfusion with 240 × 106 platelets only raised the platelet count by up to 15% of the Nbeal2−/− baseline count, but remarkably the rate of metastasis was almost restored to the levels observed in control mice. This observation makes it unlikely that the reduction in the platelet count of the Nbeal2−/− mice is responsible for the decrease in metastasis observed, but rather that it is their qualitative difference (ie, absence of a-granules that is the root cause for these results). The role of platelets in the spread of solid tumors is abundantly proven and different mechanisms have been put forward to explain this.39 Clinical implications of this phenomenon have been clearly demonstrated in a recent study showing a protective effect of low-dose “anti-platelet” aspirin for colon cancer.7 P-selectin has been shown to be a key mediator in the formation of platelet-tumor cell aggregates promoting tumor metastasis,40-43 and P-selectin–deficient mice are protected against cancer spread.44-46 The lack of P-selectin expression on the surface of activated Nbeal2−/− platelets provides a plausible explanation for the observation reported here, although we cannot exclude that other factors, such as the lack of growth factors and proangiogenic proteins normally stored in the platelet α-granules, could play a role. The majority of platelet transfusions to patients are carried out in the context of cancer treatment. The generation of platelets in vitro for clinical use is fast becoming a real prospect.47 It is not impossible to consider, informed by such models as described here, that we could genetically modify stem cells so that their platelet progeny contains granules that preserve their hemostatic function while lowering their positive effect on metastasis.48
In conclusion, we show that the pathogenicity of GPS extends beyond the absence of α-granules and a platelet hemostatic defect. We demonstrate an inflammatory component to the disease mediated, at least in part, by MKs, reflected by the development of myelofibrosis in older animals. We show evidence that Nbeal2 deficiency does not play a role in granule formation as such, but in their retention, within the MKs and platelets. Finally, we demonstrate in this mouse model for the first time, a role for platelet α-granules in the metastatic spread of solid tumors.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
The authors thank the Sanger Mouse genetics project, J.M. Sowerby for sharing samples, Jeremy Skepper for technical support on electron microscopy, and Simon McCallum for technical support on confocal microscopy.
This work was funded by the British Heart Foundation (grant FS09/039) (C.G.) and (grant RG/09/12/28096) (W.H.O. and A.R.), the National Health Service: Blood and Transplant grant (C.B. and H.M.); and the Wellcome Trust (grant WT098051) (Z.M., E.L.C., J.E., H.W.J., and A.O.S.).
Authorship
Contribution: J.A.G., C.B., L.v.d.W., H.M., M.C., P.N., A.O.S., W.N.E., and A.R. analyzed data; J.A.G., C.B., L.v.d.W., H.M., and M.C. performed experiments; J.A.G., C.B., L.v.d.W., H.M., M.C., P.N., A.O.S., W.N.E., and A.R. edited the manuscript; Z.M., E.L.C., J.E., H.W.J., and A.O.S generated the knockout mouse and prepared tissue samples; J.A.G., W.H.O., and C.G. shared writing duties; and J.A.G. and C.G. designed the study.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Jose A. Guerrero, University of Cambridge, NHS Blood and Transplant, Cambridge Blood Centre, Long Rd, CB2 0PT, Cambridge, United Kingdom; e-mail: jg652@medschl.cam.ac.uk; and Cedric Ghevaert, University of Cambridge, NHS Blood and Transplant, Cambridge Blood Centre, Long Rd, CB2 0PT, Cambridge, United Kingdom; e-mail: cg348@cam.ac.uk.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal