Key Points
MPL is essential for the development of JAK2V617F-positive myeloproliferative neoplasms in vivo.
Ablation or reduction of Mpl significantly reduces the pool of neoplastic hematopoietic stem cells.
Abstract
The most frequent contributing factor in Philadelphia chromosome-negative myeloproliferative neoplasms (MPNs) is the acquisition of a V617F mutation in Janus kinase 2 (JAK2) in hematopoietic stem cells (HSCs). Recent evidence has demonstrated that to drive MPN transformation, JAK2V617F needs to directly associate with a functional homodimeric type I cytokine receptor, suggesting that, although acquiring JAK2V617F may promote disease, there are additional cellular components necessary for MPN development. Here we show that loss of the thrombopoietin (TPO) receptor (MPL) significantly ameliorates MPN development in JAK2V617F+ transgenic mice, whereas loss of TPO only mildly affects the disease phenotype. Specifically, compared with JAK2V617F+ mice, JAK2V617F+Mpl−/− mice exhibited reduced thrombocythemia, neutrophilia, splenomegaly, and neoplastic stem cell pool. The importance of MPL is highlighted as JAK2V617FMpl+/− mice displayed a significantly reduced MPN phenotype, indicating that Mpl level may have a substantial effect on MPN development and severity. Splenomegaly and the increased neoplastic stem cell pool were retained in JAK2V617F+Tpo−/− mice, although thrombocytosis was reduced compared with JAK2V617F+ mice. These results demonstrate that Mpl expression, but not Tpo, is fundamental in the development of JAK2V617F+ MPNs, highlighting an entirely novel target for therapeutic intervention.
Introduction
The hematopoietic cytokine thrombopoietin (TPO) is the primary regulator of megakaryopoiesis, driving progenitor expansion and early stages of megakaryocyte differentiation.1-3 Additionally, TPO is critical for hematopoietic stem cell (HSC) survival and proliferation.4 Inactivating mutations of MPL, the receptor for TPO, leads to congenital amegakaryocytic thrombocytopenia (CAMT) and eventual aplastic anemia as a result of a reduced HSC pool.5,6 Conversely, activating mutations of MPL are responsible for the development of essential thrombocythemia (ET) and primary myelofibrosis (PMF) in a small number of patients.7-9 Similar to a number of other type I cytokine receptors, MPL lacks intrinsic kinase activity, instead relying on interactions with the nonreceptor tyrosine kinase Janus kinase 2 (JAK2). TPO binding to MPL results in the rapid cross-phosphorylation and activation of JAK2, which in turn leads to MPL phosphorylation and activation of numerous downstream signaling pathways involving signal transducers and activators of transcription (STAT)1, 3 and 5, Akt and extracellular response kinase (ERK)1/2.10
Myeloproliferative neoplasms (MPNs) are a group of diseases that includes polycythemia vera (PV), ET, and PMF and are characterized by hyperproliferation of cells of the myeloid lineage. Activating mutations of MPL are found in the neoplastic cells of 5% to 10% of patients with ET or PMF.7-9 The acquired JAK2V617F mutation is the most common mutation in MPNs, identified in the blood cells of nearly all patients with PV and ∼50% of patients with ET and PMF.11-14 In vitro studies demonstrated that JAK2V617F is hypersensitive and is able to initiate signaling in the absence of, or at very low concentrations of cytokine.15-17 Mutations in other genes such as tet methylcytosine dioxygenase 2 (TET2), additional sex combs 1 (ASXL1), SH2B3 (LNK),18 and calreticulin (CALR)19,20 also contribute to the development of JAK2V617F-positive MPNs, whereas allelic burden of the mutant JAK2 also has significant effects on disease phenotype and prognosis.21,22 The efficacy of therapeutic JAK2 inhibitors has been limited due to specificity issues and adverse side effects, because JAK2 function is critical for normal hematopoiesis. Although 1 drug, INCB018424 (ruxolitinib), is now in clinical use for the reduction of splenomegaly resulting from PMF, results are, at best, mixed.23,24 Consequently, the identification of alternative targets for the development of more effective MPN therapies would be extremely beneficial.
The importance of TPO/MPL signaling in MPN development has been suggested previously. Work by Li et al demonstrated that reducing MPL expression using antisense oligonucleotides significantly reduced spontaneous colony-forming unit megakaryocyte (CFU-MK) formation in patients with ET and PV,25 whereas ectopic overexpression of MPL induced a severe PV-like disease in mice.26 Additionally, attenuated MPL expression has been observed in platelets isolated from PV patients,27 although it is not clear whether this finding contributes to disease phenotype or is a result of JAK2V617F expression. More recent in vitro studies demonstrate that expression of a functional, membrane-localized homodimeric receptor is critical in JAK2V617F-mediated hyperproliferation in cell line models. Lu and colleagues demonstrated that expression of a type I homodimeric cytokine receptor is a vital for JAK2V617F-mediated cytokine independent growth and the initiation of JAK/STAT signaling.15 It should be noted that of the 3 homodimeric cytokine receptors studied, only MPL is expressed in HSCs, making it a likely target of driver mutations for the MPNs. Additionally, mutating the protein 4.1, ezrin, radixin, moesin domain of JAK2V617F, the region that directly associates with box1/2 domains of homodimeric receptors, prevented growth factor independent cell growth and signaling in erythropoietin receptor (EPOR)-expressing cell lines.16 Taken together, these data suggest that interactions between a homodimeric receptor and JAK2V617F are essential for the development of growth factor independence in culture, which is the in vitro correlate of myeloproliferation in vivo. However, the roles of specific type I cytokines and their receptors in MPN development in vivo is yet to be proven.
In this study, we determined the roles of TPO and MPL in the development of JAK2V617F-positive MPNs in vivo by ablating Tpo or Mpl in a previously described MPN mouse model (FF1/Tie2-Cre).28
Methods
JAK2V617F Flip-Flop (FF1) mice21 were kindly provided by Radek Skoda (University Hospital, Basal, Switzerland), Tie2-Cre mice29 by Mark Ginsberg (University of California, San Diego, CA), Mpl knockout mice (Mpl−/−)30 by Warren Alexander (Melbourne, Australia), and Tpo knockout mice31 (Tpo−/−) by Fred de Sauvage at Genentech (South San Francisco, CA). All strains were bred onto the same genetic background (C57BL/6) and bred in a pathogen-free mouse facility at Stony Brook University (New York, NY). CD45.1+ congenic mice (SJL) were purchased from Jackson Laboratories. FF1/Tie2-Cre (JAK2V617F+) mice expressing JAK2V617F in HSCs were generated as previously described.28 To generate JAK2V617F expressing mice lacking Mpl or Tpo, JAK2V617F+ mice were crossed with Mpl−/− or Tpo−/− mice to generate JAK2V617F+Mpl+/−, JAK2V617F+Mpl−/−, JAK2V617F+Tpo−/− mice and littermate controls. Animal experiments were performed in accordance with the guidelines provided by the Institutional Animal Care and Use Committee at Stony Brook University.
Additional methods can be found in the supplemental Methods, available on the Blood Web site.
Results
Mpl and Tpo are necessary for development of JAK2V617F-positive MPNs
Transgenic mice expressing human JAK2V617F28 were studied in a Mpl homozygous null (JAK2V617F+Mpl−/−), Mpl heterozygous (JAK2V617F+Mpl+/−), or Tpo homozygous null (JAK2V617F+Tpo−/−) background. Platelet MPL expression was halved in Mpl heterozygous (Mpl+/−) mice compared with Mpl homozygous wild-type mice (WT) (supplemental Figure 1). Additionally, JAK2V617F expression resulted in increased Mpl expression (supplemental Figure 1). Levels of JAK2V617F cDNA ranged from 10% to 50% of that of the endogenous murine JAK2,28 which is close to that expected in blood cells of patients with MPNs expressing a single copy of the mutant kinase.
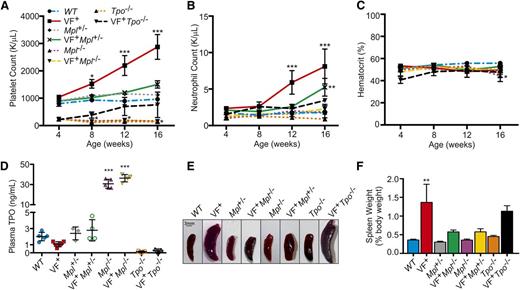
JAK2V617F expression in HSCs resulted in MPN development by 12 weeks of age with a significant increase in platelet (Figure 1A) and neutrophil count (Figure 1B) but no change in hematocrit or red blood cell count (Figure 1C; supplemental Figure 2) compared with WT.
Removal of Mpl or Tpo in JAK2V617F+ MPN mouse models rescues normal hematopoiesis. (A) Platelet, (B) neutrophil counts, and (C) hematocrit were monitored for 16 weeks after birth in WT, JAK2V617F+ (VF+), JAK2V617F+Mpl+/− (VF+Mpl+/−), JAK2V617F+Mpl−/− (VF+Mpl−/−), and JAK2V617F+Tpo−/− (VF+Tpo−/−) mice. Data are presented as mean ± standard error of the mean (SEM); n = 3 to 9 mice per time point. (D) Plasma TPO levels in Mpl and Tpo transgenic mice. Plasma was collected from 16-week-old WT, JAK2V617F+ (VF+), Mpl+/−, JAK2V617F+Mpl+/− (VF+Mpl+/−), Mpl−/−, JAK2V617F+Mpl−/− (VF+Mpl−/−), Tpo−/−, and JAK2V617F+Tpo−/− mice, and TPO levels were quantified by enzyme-linked immunosorbent assay. Each data point represents a single mouse, and bars are presented as mean ± SEM; asterisks indicate significance compared with WT. (E) Representative spleens and (F) weights of spleens harvested from 16-week-old mice. Scale bar, 3 mm.
Removal of Mpl or Tpo in JAK2V617F+ MPN mouse models rescues normal hematopoiesis. (A) Platelet, (B) neutrophil counts, and (C) hematocrit were monitored for 16 weeks after birth in WT, JAK2V617F+ (VF+), JAK2V617F+Mpl+/− (VF+Mpl+/−), JAK2V617F+Mpl−/− (VF+Mpl−/−), and JAK2V617F+Tpo−/− (VF+Tpo−/−) mice. Data are presented as mean ± standard error of the mean (SEM); n = 3 to 9 mice per time point. (D) Plasma TPO levels in Mpl and Tpo transgenic mice. Plasma was collected from 16-week-old WT, JAK2V617F+ (VF+), Mpl+/−, JAK2V617F+Mpl+/− (VF+Mpl+/−), Mpl−/−, JAK2V617F+Mpl−/− (VF+Mpl−/−), Tpo−/−, and JAK2V617F+Tpo−/− mice, and TPO levels were quantified by enzyme-linked immunosorbent assay. Each data point represents a single mouse, and bars are presented as mean ± SEM; asterisks indicate significance compared with WT. (E) Representative spleens and (F) weights of spleens harvested from 16-week-old mice. Scale bar, 3 mm.
Despite JAK2V617F expression, in the complete absence of Mpl (JAK2V617F+Mpl−/−) the mice remained thrombocytopenic, with platelet levels similar to that of Mpl−/− controls (Figure 1A). When Mpl expression was halved (JAK2V617F+Mpl+/−), platelet counts were significantly decreased compared with JAK2V617F+ controls, to levels comparable with WT mice (Figure 1A). Neutrophil counts were also reduced in JAK2V617F+Mpl+/− compared with JAK2V617F+ mice, but were significantly higher than WT controls at 16 weeks (Figure 1B). Tpo ablation (JAK2V617F+Tpo−/−) also significantly decreased platelet number compared with JAK2V617F+ controls. However, expression of JAK2V617F was able to increase platelet counts over time to levels comparable to WT levels (Figure 1A). Neutrophil counts, hematocrit, and red blood cell counts in JAK2V617F+Tpo−/− mice were not significantly different from WT (Figure 1B-C; supplemental Figure 3).
The uptake and destruction of TPO by platelet MPL is a major regulator of circulating TPO blood levels.32 As expected, the thrombocythemia observed in JAK2V617F+ mice resulted in reduced TPO plasma levels, whereas Mpl−/− and JAK2V617F+Mpl−/− mice showed significantly increased plasma TPO levels (Figure 1D). Mpl+/− and JAK2V617F+Mpl+/− mice show a slight increase compared with WT and JAK2V617F+ mice and Tpo−/− mice are completely TPO deficient (Figure 1D). Increased bioavailability of TPO may provide a potential explanation as to why the JAK2V617F+Mpl+/− mice display a moderate increase in platelet and neutrophil counts at 16 weeks (Figure 1A-B) and is also demonstrated by the increased baseline platelet count in Mpl+/− mice compared with Mpl−/− mice.
We previously reported that this JAK2V617F+ mouse model develops splenomegaly with abnormal splenic architecture, elevated number of megakaryocytes, and significant expansion of primitive (TER-119+ CD71+) and more mature (TER-119+ CD71−) erythroblasts in the spleen.28 In the absence of, or with reduced expression of, Mpl, splenomegaly was significantly reduced (P = .0166 and P = .0181, respectively, compared with JAK2V617F+ by 1-way analysis of variance [ANOVA]; Figure 1E-F). Removal of Mpl (JAK2V617F+Mpl−/−) significantly decreased the number of splenic megakaryocytes (supplemental Figure 4A-B) and splenic erythrocyte expansion was reduced in JAK2V617F+Mpl−/− and JAK2V617F+Mpl+/− mice (supplemental Figure 5). Conversely, removal of Tpo was not effective in reducing spleen size and weight as evidenced in our JAK2V617F+Tpo−/− mice (Figure 1E-F). Splenic megakaryocytes were more prevalent in JAK2V617F+Tpo−/− compared with JAK2V617F+ mice, although average megakaryocyte size was reduced (supplemental Figure 4A-C). We also observed an expansion of splenic TER-119+ CD71+ erythroid progenitors in these mice (supplemental Figure 5).
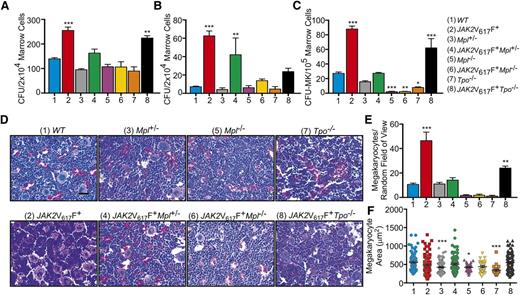
Bone marrow progenitor cells were analyzed by colony forming unit (CFU) assays in the presence or absence of cytokine. In WT mice, the expression of JAK2V617F significantly increased the number of cytokine-dependent (Figure 2A) and cytokine-independent (Figure 2B) marrow progenitor cells. However, removal of Mpl (JAK2V617F+Mpl−/−) resulted in CFU numbers being dramatically reduced both in the presence or absence of cytokine to levels not significantly different to those found in WT marrow (Figure 2A-B). Consistent with the observed thrombocytopenia, JAK2V617F+Mpl−/− mice exhibited an almost complete ablation of megakaryocyte progenitors, CFU-MK (Figure 2C). A similar phenotype was observed in factor-dependent colony formation in marrow from JAK2V617F+Mpl+/− mice (Figure 2A), although in the absence of cytokine, CFU numbers were similar to JAK2V617F+ controls (Figure 2B), potentially accounting for the neutrophilia observed in these mice at 16 weeks (Figure 1B). CFU-MK in JAK2V617F+Mpl+/− mice and Mpl+/− controls were not significantly different to WT (Figure 2C). In the absence of cytokine, CFU numbers for Tpo−/− and JAK2V617F+Tpo−/− mice were significantly reduced compared with JAK2V617F+ controls (P < .0001 and P = .0014, respectively, compared with JAK2V617F+ by 1-way ANOVA; Figure 2B). Megakaryocyte progenitors of JAK2V617F+Tpo−/− mice were significantly increased compared with WT; however, there were still significantly less CFU-MK compared with JAK2V617F+ mice (P = .0013 compared with JAK2V617F+ by 1-way ANOVA; Figure 2C). Of note, the observed reduction/elimination of JAK2V617F-induced pathology was achieved despite expression of comparable levels of mutant JAK2 in all of the JAK2V617F+ mouse lines (supplemental Figure 6).
Mpl is essential for the development of JAK2V617F+ MPNs. Data were collected from 16-week-old WT, JAK2V617F+, Mpl+/−, JAK2V617F+Mpl+/−, Mpl−/−, JAK2V617F+Mpl−/−, Tpo−/−, and JAK2V617F+Tpo−/− mice. (A) Number of progenitor cells present per 2 × 104 marrow cells as determined by CFU assays in the presence and (B) absence of cytokine. Data are presented as mean ± SEM; n = 3 to 5. (C) CFU-MK per 1 × 105 marrow cells as determined by Megacult assays in the presence of cytokine. Data are presented as mean ± SEM. n = 3 to 5. (D) Representative hematoxylin and eosin-stained sections of bone marrow with (E) megakaryocyte count and (F) size. Scale bar, 50 μm.
Mpl is essential for the development of JAK2V617F+ MPNs. Data were collected from 16-week-old WT, JAK2V617F+, Mpl+/−, JAK2V617F+Mpl+/−, Mpl−/−, JAK2V617F+Mpl−/−, Tpo−/−, and JAK2V617F+Tpo−/− mice. (A) Number of progenitor cells present per 2 × 104 marrow cells as determined by CFU assays in the presence and (B) absence of cytokine. Data are presented as mean ± SEM; n = 3 to 5. (C) CFU-MK per 1 × 105 marrow cells as determined by Megacult assays in the presence of cytokine. Data are presented as mean ± SEM. n = 3 to 5. (D) Representative hematoxylin and eosin-stained sections of bone marrow with (E) megakaryocyte count and (F) size. Scale bar, 50 μm.
Histological analysis of bone marrow sections showed a dramatic increase in the number of megakaryocytes in JAK2V617F+ mice compared with WT, an effect that was completely ablated in JAK2V617F+Mpl+/− and JAK2V617F+Mpl−/− mice (Figure 2D-E). Similar to our previous results, JAK2V617F+Mpl+/− and JAK2V617F+Mpl−/− appeared analogous to WT and Mpl−/−, respectively. JAK2V617F+Tpo−/− mice showed an increase in the number of megakaryocytes compared with WT, which is consistent with the increase in CFU-MK. However, mature marrow megakaryocyte number was still significantly less than in JAK2V617F+ marrow (P < .001 compared with JAK2V617F+ by 1-way ANOVA; Figure 2D-E). In the absence or reduction of Mpl and absence of Tpo, megakaryocyte size was significantly reduced (Figure 2F), consistent with previous reports.31 This effect was overcome by presence of JAK2V617F (Figure 2F). Despite increases in megakaryocyte number in bone marrow of these mice, there was no detectable fibrosis of the marrow (supplemental Figure 7).
Taken together, these data clearly demonstrate that MPL is essential in MPN progression. Specifically, the JAK2V617F-mediated MPN in this murine model fails to develop in the absence of Mpl; however, the disease was only nominally attenuated in the absence of Tpo. Importantly, we also demonstrate that reducing Mpl expression significantly attenuates MPN progression in JAK2V617F+ mice, indicating that Mpl is a limiting factor for JAK2V617F-driven MPNs.
Attenuation of JAK2V617F-positive MPNs through removal of Tpo or Mpl is not a result of dysfunctional primitive hematopoiesis
Mpl is expressed in primitive HSCs during embryogenesis and is also critical in the establishment of normal adult hematopoiesis.33 To ensure that the effects we observed in the JAK2V617F+Mpl−/− mice were not a result of dysfunctional primitive hematopoiesis, we performed a series of transplantation experiments using JAK2V617F+ and JAK2V617F+Mpl−/− adult donor marrow cells. This allowed us to specifically determine the consequences of introducing the JAK2V617F mutation during adulthood, therefore better reflecting the development of the human disease through acquisition of a somatic mutation in JAK2.
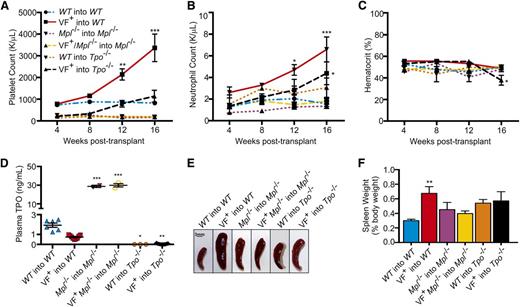
Due to its critical role in hematopoiesis, Mpl−/− marrow has severely decreased repopulating activity.34 Therefore, to ensure that the observed effects were due to engrafted donor marrow and not due to recovery of recipient marrow, donor cells lacking Mpl were transplanted into Mpl−/− recipients rather than WT recipients. To generate a JAK2V617F+Tpo−/− transplant model, JAK2V617F+Tpo+/+ marrow cells were transplanted into Tpo−/− recipients. As TPO is synthesized primarily in the liver, transplantation into a Tpo−/− background results in a functionally Tpo−/− mouse, although it is theoretically possible that engraftment of stromal cells from the JAK2V617F+Tpo+/+ marrow could supply a very low level of TPO in our mouse model. In control mice, chimerism was confirmed at 4 weeks, and periodic monitoring verified it was maintained at an average of 95% ± standard deviation. Similar to the congenital JAK2V617F+ mutants, WT recipient mice transplanted with JAK2V617F+ marrow developed a MPN by 12 weeks after transplant, shown by increased platelet (Figure 3A) and neutrophil counts (Figure 3B). However, transplantation of JAK2V617F+Mpl−/− donor marrow (VF+Mpl−/−into Mpl−/−) failed to drive the development of a MPN (Figure 3A-B). Such transplant mice remained severely thrombocytopenic, whereas neutrophil, hematocrit, and red blood cells counts were not significantly different to WT (Figure 3A-C; supplemental Figure 8). In a Tpo−/− background, introduction of JAK2V617F by transplantation (VF+ into Tpo−/−) was unable to generate a severe MPN, but did drive platelet production to levels comparable with WT (Figure 3A-C; supplemental Figure 9). The increased platelet count was accompanied by an increase in neutrophil count at 16 weeks after transplant (Figure 3B).
In marrow transplantation models of JAK2V617F+ MPNs, Mpl and Tpo are required for aberrant hematopoiesis. (A) Platelet, (B) neutrophil counts, and (C) hematocrit from WT into WT, JAK2V617F+ into WT (VF+ into WT), Mpl−/− into Mpl−/−, JAK2V617F+Mpl−/− into Mpl−/− (VF+ into Mpl−/−), WT into Tpo−/−, and JAK2V617F+ into Tpo−/− transplant mice were monitored for 16 weeks after transplant. Data are presented as mean ± SEM; n = 2 to 10 mice per time point. (D) Plasma was collected 16 weeks after transplant from WT into WT, JAK2V617F+ into WT (VF+ into WT), Mpl−/− into Mpl−/−, JAK2V617F+Mpl−/− into Mpl−/− (VF+ into Mpl−/−), WT into Tpo−/−, and JAK2V617F+Tpo−/− mice, and TPO levels were quantified by enzyme-linked immunosorbent assay. Each data point represents a single mouse, and bars are presented as mean ± SEM; asterisks indicate significance compared with WT into WT. (E) Representative spleens and (F) weights of spleens harvested 16 weeks after transplant. Scale bar, 3 mm.
In marrow transplantation models of JAK2V617F+ MPNs, Mpl and Tpo are required for aberrant hematopoiesis. (A) Platelet, (B) neutrophil counts, and (C) hematocrit from WT into WT, JAK2V617F+ into WT (VF+ into WT), Mpl−/− into Mpl−/−, JAK2V617F+Mpl−/− into Mpl−/− (VF+ into Mpl−/−), WT into Tpo−/−, and JAK2V617F+ into Tpo−/− transplant mice were monitored for 16 weeks after transplant. Data are presented as mean ± SEM; n = 2 to 10 mice per time point. (D) Plasma was collected 16 weeks after transplant from WT into WT, JAK2V617F+ into WT (VF+ into WT), Mpl−/− into Mpl−/−, JAK2V617F+Mpl−/− into Mpl−/− (VF+ into Mpl−/−), WT into Tpo−/−, and JAK2V617F+Tpo−/− mice, and TPO levels were quantified by enzyme-linked immunosorbent assay. Each data point represents a single mouse, and bars are presented as mean ± SEM; asterisks indicate significance compared with WT into WT. (E) Representative spleens and (F) weights of spleens harvested 16 weeks after transplant. Scale bar, 3 mm.
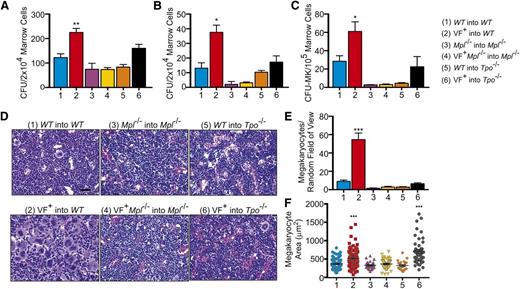
Splenomegaly present in the JAK2V617F+ into WT transplant mice was not observed in the JAK2V617F+Mpl−/− into Mpl−/− mice or JAK2V617F+ into Tpo−/− transplants (Figure 3E-F). Additionally, consistent with the blood counts, colony assays in the presence of cytokines demonstrated a significant increase in JAK2V617F+ into WT bone marrow progenitors compared with WT into WT, JAK2V617F+Mpl−/− into Mpl−/−, and V617F+ into Tpo−/− mice (Figure 4A). The effect on the development of progenitor cells was even more apparent when cells were cultured in the absence of cytokine, with an ∼10-fold decrease in the number of colonies developing from marrow derived from JAK2V617F+Mpl−/− into Mpl−/− transplant mice compared with marrow from JAK2V617F+ into WT controls (Figure 4B). In the absence of cytokine, JAK2V617F+ into Tpo−/− mice show a significant decrease in the number of colonies that developed compared with JAK2V617F+ into WT animals (P = .0068; Figure 4B).
Mpl is required for development of JAK2V617F+ MPNs in marrow transplantation models. Data were collected from WT into WT, JAK2V617F+ into WT (VF+ into WT), Mpl−/− into Mpl−/−, JAK2V617F+Mpl−/− into Mpl−/− (VF+ into Mpl−/−), WT into Tpo−/−, and JAK2V617F+ into Tpo−/− mice 16 weeks after transplant. (A) Number of progenitor cells present per 2 × 104 marrow cells as determined by CFU assays in the presence and (B) absence of cytokine. Data are presented as mean ± SEM; n = 2 to 12. (C) CFU-MK per 1 × 105 marrow cells as determined by Megacult assays in the presence of cytokine. Data are presented as mean ± SEM. n = 2 to 12. (D) Representative hematoxylin and eosin-stained sections of bone marrow with (E) megakaryocyte count and (F) size. Scale bar, 50 μm.
Mpl is required for development of JAK2V617F+ MPNs in marrow transplantation models. Data were collected from WT into WT, JAK2V617F+ into WT (VF+ into WT), Mpl−/− into Mpl−/−, JAK2V617F+Mpl−/− into Mpl−/− (VF+ into Mpl−/−), WT into Tpo−/−, and JAK2V617F+ into Tpo−/− mice 16 weeks after transplant. (A) Number of progenitor cells present per 2 × 104 marrow cells as determined by CFU assays in the presence and (B) absence of cytokine. Data are presented as mean ± SEM; n = 2 to 12. (C) CFU-MK per 1 × 105 marrow cells as determined by Megacult assays in the presence of cytokine. Data are presented as mean ± SEM. n = 2 to 12. (D) Representative hematoxylin and eosin-stained sections of bone marrow with (E) megakaryocyte count and (F) size. Scale bar, 50 μm.
In JAK2V617F+Mpl−/− into Mpl−/−mice, CFU-MK were depleted compared with JAK2V617F+ into WT controls (Figure 4C), again demonstrating the importance of Mpl in JAK2V617F+ MPNs. In WT into Tpo−/− mice, CFU-MK were similarly depleted; however, in JAK2V617F+ into Tpo−/− mice, counts were comparable with WT into WT controls (Figure 4C). Consistent with the high platelet count, histological examination of JAK2V617F into WT showed greatly elevated numbers of megakaryocytes present in the bone marrow (Figure 4D-E). However, this effect was absent in JAK2V617F+Mpl−/− into Mpl−/− and JAK2V617F+ into Tpo−/− transplants (Figure 4D-E). Megakaryocytes from JAK2V617F+ mice were larger than WT, consistent with the higher platelet count in these mice (Figure 4F). However, despite reduced numbers of megakaryocytes in JAK2V617F+Tpo−/− mice, their megakaryocytes were also larger compared with WT, which may explain the gradual increase in platelet count (Figure 4F). Similar to results from our congenital mouse models, plasma TPO levels were high in mice with an Mpl−/− background and absent in Tpo−/− mice (Figure 3D). Together, these data demonstrate that Tpo plays a minimal role in JAK2V617F+ MPN development, whereas Mpl expression in HSCs is absolutely required for JAK2V617F-mediated transformation. Additionally, introduction of JAK2V617F during adulthood is sufficient for development of an MPN, but in this setting also, the disease is partially dependent on Tpo expression and entirely dependent on expression of Mpl.
JAK2V617F promotion of HSC expansion is dependent on Mpl expression
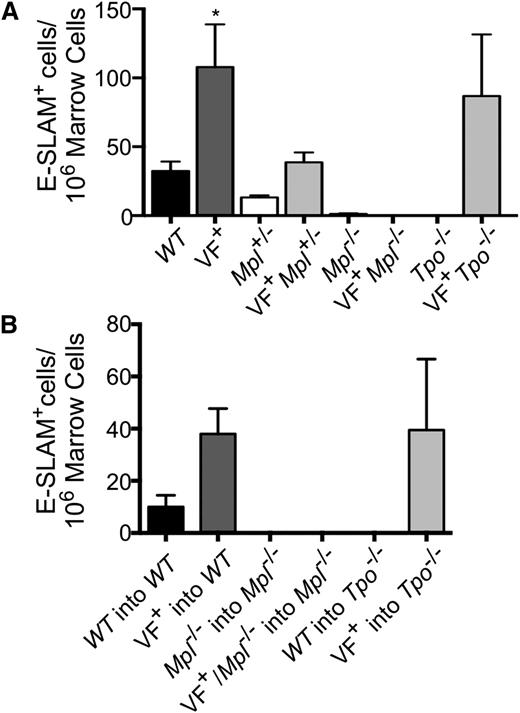
Given that the JAK2V617F mutation is acquired in HSCs and the importance of both Tpo and Mpl in HSC maintenance, we determined whether the observed differences in pathology in our mouse models were due to a change in the number of primitive HSCs. Therefore, E-SLAM+ [CD45+EPCR(CD201)+CD48−CD150+] cells, a highly purified population of long-term repopulating HSCs,35 were enumerated in marrow (Figure 5A-B; supplemental Figure 10). In our congenital models of MPNs, we found that JAK2V617F expression significantly increased the number of E-SLAM+ HSCs compared with WT (Figure 5A). Mpl ablation resulted in a greatly decreased E-SLAM+ population, both in the presence and absence of JAK2V617F (Figure 5A). However, JAK2V617F+Mpl+/− mice showed reduced numbers of E-SLAM+ cells compared with JAK2V617F+ controls to a level similar to WT, demonstrating that both JAK2V617F and Mpl expression are important for the pathologic increase of HSCs observed in MPNs (Figure 5A). Conversely, E-SLAM+ numbers in JAK2V617F+Tpo−/− mice are comparable to JAK2V617F+ controls; thus, Tpo is not necessary for JAK2V617F to promote HSC expansion (Figure 5A). Mpl expression was also found to be critical in expanding the marrow E-SLAM+ populations in our transplant models of MPNs, whereas removal of Tpo had little or no effect (Figure 5B). Our data indicate that JAK2V617F significantly increases the pool of E-SLAM+ HSCs, likely promoting MPN development. These data show that the proliferative effect of JAK2V617F on HSCs is partially dependent on Tpo expression and entirely dependent on Mpl expression. The decreased number of HSCs associated with Mpl reduction or ablation may provide a potential mechanism through which the absence of Mpl prevents MPN development.
Mpl is necessary for primitive HSC overexpansion observed in JAK2V617F+ MPNs. (A) Bone marrow cells harvested from 16-week-old congenital or (B) transplant mice 16-weeks after transplant were analyzed for number of E-SLAM+ HSCs by flow cytometry. Data are presented as mean ± SEM; n = 2 to 9 mice per group.
Mpl is necessary for primitive HSC overexpansion observed in JAK2V617F+ MPNs. (A) Bone marrow cells harvested from 16-week-old congenital or (B) transplant mice 16-weeks after transplant were analyzed for number of E-SLAM+ HSCs by flow cytometry. Data are presented as mean ± SEM; n = 2 to 9 mice per group.
Discussion
TPO and MPL are well known for their role in HSC maintenance and the regulation of platelet production.36-38 Like many type I cytokine receptors, MPL lacks intrinsic kinase activity and relies on JAK2 for signal transduction. Activating mutations in MPL cause ET, and recent experimental data show that JAK2 is required for this process,39 further highlighting the interdependency of MPL and JAK2. As MPNs are a stem cell disorder and given the role of MPL and TPO in HSC homeostasis and the dependency of MPL on JAK2, here we investigated the role of TPO and MPL in JAK2V617F+ MPNs.
In addition to MPL, JAK2 is also responsible for signal transduction of the EPOR, Granulocyte colony-stimulating factor receptor, interleukin-3 receptor, and stem cell factor receptor.40-42 Thus, it was possible that removal of Mpl could result in a differential receptor interaction of JAK2. However, modulation of Mpl or Tpo expression resulted in changes to the severity of the MPN compared with our JAK2V617F+ control model, but did not affect the type of MPN developed. For example, the drop in platelet and neutrophil counts resulting from modulation of Mpl or Tpo expression was not accompanied by an increase in hemoglobin, red cell count, or hematocrit, which would be indicative of PV. This finding would suggest that MPL is critical in the oncogenic transformation of HSCs in JAK2V617F-positive MPNs. As the effect of MPL modulation is observed at the HSC level, we postulate that reduction or removal of MPL in a PV mouse model will also ameliorate the disease. However, as our mouse model recreates a disease similar to ET, future investigations are required to see if MPL is necessary for the development of other JAK2V617F+ MPNs such as PV and PMF.
Although complete Mpl ablation in our model prevented MPN development, it resulted in thrombocytopenia. This is recapitulated in humans that lack functional MPL, resulting in defective responses to TPO causing CAMT.6 However, in CAMT patients, unlike our mouse models, thrombocytopenia progresses to pancytopenia, and these patients require a bone marrow transplant, making complete removal of MPL an implausible means for therapeutic intervention. Nevertheless, our studies highlight the critical importance of Mpl in JAK2V617F+ MPN development in vivo. Indeed the necessity of MPL is highlighted in our JAK2V617FMpl+/−model, as reducing Mpl expression resulted in severely attenuated MPN progression, with platelet levels comparable to WT. Additionally, whereas HSCs were dramatically reduced in JAK2V617F+Mpl−/− mice, expression of half the amount of Mpl in conjunction with JAK2V617F rescues the HSC pool.
Recent reports have provided new insights into the roles of TPO/MPL/JAK2 in megakaryopoiesis. Ng and colleagues have shown that knocking out Mpl specifically in megakaryocytes and platelets caused myeloproliferation as a result of increased TPO availability.43 In addition, Meyer et al demonstrated that ablation of Jak2 specifically in megakaryocytes resulted in stem and progenitor cell expansion and thrombocytosis, presumably due to reduced internalization and degradation of the TPO/MPL complex in the absence of JAK2.44 Similarly, using UT7-MPL cell lines, Besancenot et al showed that low levels of MPL expression increased TPO-stimulated proliferation, whereas low doses of JAK2 chemical inhibitors caused increased platelet production in mice.45 It would appear that some of these data contradict our findings that reduced Mpl expression prevents JAK2V617F-mediated myeloproliferation. However, there are a number of differences between the studies that may explain the varying results. In our study, we reduced or ablated Mpl expression throughout hematopoiesis, not just in megakaryocytes or platelets as in the studies above. Therefore, in our system, the increases in circulating TPO caused by reduced platelet MPL expression fails to stimulate myeloproliferation due to the reduction or absence of MPL in hematopoietic stem and progenitor cells. It would also appear that there are significant differences between the roles of TPO/MPL in megakaryopoiesis and their roles in hematopoietic progenitor expansion. Whereas the data presented by Meyer et al and Besancenot et al specifically investigate the function of MPL in megakaryopoiesis in vivo or megakaryocytic cell lines in vitro, we focused more on the roles of MPL/JAK2V617F in neoplastic progenitor cell expansion. Taken together, these data suggest that our understanding of TPO/MPL/JAK2 in hematopoiesis is still evolving, and the biphasic roles of MPL should be taken into account when targeting MPL therapeutically.
Several groups have shown that expression of MPL or EPOR in conjunction with JAK2V617F in cell lines was sufficient to stimulate proliferation in the absence of cytokines,15,16,46 suggesting that the presence of JAK2V617F and a receptor scaffold is sufficient for disease development. Although reducing Mpl had a far more significant effect on disease progression, it was surprising and contradictory to previous in vitro findings that Tpo ablation attenuated several aspects of MPN development. Despite demonstrating that JAK2V617F and Mpl are sufficient for MPN development, our data also suggest that in the presence of TPO, the disease progression, specifically thrombocythemia and neutrophilia, is greatly accelerated. However, although at 16 weeks JAK2V617F+Tpo−/− mice have platelet counts similar to WT, it is possible that these mice will eventually develop thrombocythemia as platelet counts are conceivably still rising. Additionally, JAK2V617F-mediated splenomegaly and expansion of the HSC pool was not affected by the removal of TPO. We postulate that the unresolved splenomegaly in our JAK2V617F+Tpo−/− mice is a consequence extramedullary erythrocytosis29 and megakaryocyte hyperplasia. The number of neoplastic HSCs in JAK2V617F+Tpo−/− mice is similar to JAK2V617F+, suggesting that in the absence of TPO, JAK2V617F is still able to exert a proliferative effect on HSCs. Therefore, it is likely that Mpl serves as a scaffold for JAK2V617F and is sufficient for initiation of JAK2/STAT signaling in the absence of cytokine as previously suggested in vitro.15,16,46
Interestingly, we found that MPL protein expression was increased in platelets from JAK2V617F+ and JAK2V617F+Mpl+/− mice. These results are contradictory to previous findings with an alternative JAK2V617F knock-in mouse model.47 It is not clear why the mouse models express different levels of MPL, but it should be noted that they exhibit significantly different phenotypes. Although our FF1/Tie2-Cre mice present with thrombocytosis, neutrophilia, and splenomegaly, the mice used by Pecquet et al (which were originally generated by Marty and colleagues48 ) also develop high red blood cell counts, leukocytosis, and myelofibrosis. Whether different disease phenotypes alter MPL expression is not currently known, but given our data demonstrating the significance of MPL levels in disease progression, this is an important subject for future investigation.
JAK2V617F expression increases the number of E-SLAM+ HSCs, an effect that is severely attenuated by Mpl ablation. The increase in the HSC pool in JAK2V617F+Tpo−/− and JAK2V617F+Mpl+/− mice compared with WT could explain the delayed increase in neutrophils at 16 weeks. Whether or not JAK2V617F gives HSCs a selective advantage remains controversial.49 Our data support previous reports that the JAK2V617F mutation expands the HSC pool,50 although discrepancies may be due to significant differences between mouse models, which develop various disease phenotypes over different periods of time.51
In this report, we not only demonstrate that MPL is necessary for the development of a JAK2V617F-mediated MPN in transgenic mouse models, but that the level of receptor expression directly affects disease severity and progression. We show that the JAK2V617F oncogene requires the MPL transmembrane receptor to initiate the hematological malignancy in HSCs and that TPO contributes to disease progression but is not necessary for MPN development. The use of JAK2 inhibitors, such as ruxolitinib, has now been approved by the Foody and Drug Administration to treat splenomegaly associated with myelofibrosis. However, clinical response to the drug has been mixed, warranting the exploration of other drug targets. Complete ablation of TPO only exerted a minor effect on MPN development; therefore, targeting of TPO in MPNs would likely require complete inhibition, making TPO a difficult target for clinical drug development. Instead the data presented here highlight MPL as an excellent therapeutic target for the treatment of MPNs. Small molecule MPL agonists (eltrombopag) and TPO peptidomimetics (romiplostim) are now in clinical use for the treatment of idiopathic thrombocytopenic pupura, clearly demonstrating the feasibility of targeting drugs to modulate MPL activity. We propose that modifying MPL levels through the use of anitbodies, small molecule inhibitors, or peptide mimetics or preventing its association with TPO in the presence of JAK2V617F may alter the clinical and pathological manifestations of MPNs.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
The authors thank the Research Histology Core Laboratory at Stony Brook Medicine for assistance with our histological studies.
This research was supported by National Institutes of Health, National Institute of Diabetes and Digestive and Kidney Diseases grant 2R01DK049855-15A (to K.K. and I.S.H.).
Authorship
Contribution: V.S. designed and performed experiments, analyzed data, and wrote the manuscript; S.L.E. performed experiments, analyzed data, and wrote the manuscript; K.K. analyzed data and wrote the manuscript; and I.S.H. designed and performed experiments, analyzed data, wrote the manuscript, and supervised the project.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Ian S. Hitchcock, Centre for Immunology and Infection, Department of Biology, University of York, Heslington, York YO10 5DD, United Kingdom; e-mail: ian.hitchcock@york.ac.uk.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal