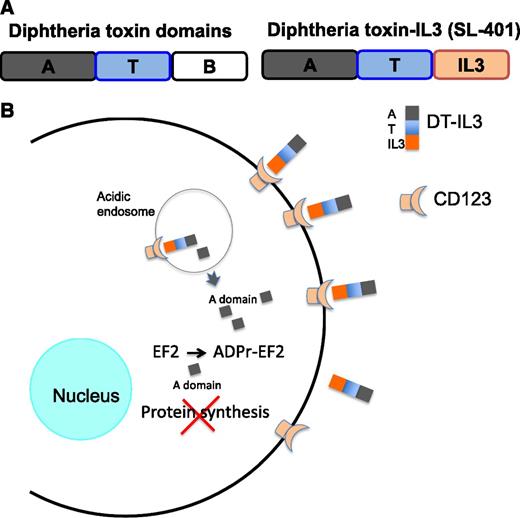
In this issue of Blood, Frankel et al describe a novel treatment of blastic plasmacytoid dendritic cell neoplasm (BPDCN) using an engineered version of diphtheria toxin that is targeted to malignant cells via a fusion with interleukin (IL)3 (see panel A).1
(A) The fusion of IL3 at the C terminus of the truncated diphtheria toxin. The binding domain of diphtheria toxin, located at the C terminus, is deleted and replaced by IL3. Thus, the cytotoxicity of the fusion protein is targeted to cells expressing IL3 receptors (CD123). The domain names are as follows: A, active enzyme domain; T, translocation domain; B, binding domain. The binding domain is removed and replaced by IL3 to form DT-IL3, which is called SL-401 in Frankel et al.1 (B) The pathway of binding, entry, and killing by DT-IL3. DT-IL3 binds CD123 and is internalized, and the A chain is processed proteolytically to release the A chain. The A chain translocates from acidic endosomes to the cytosol. In the cytosol, the A chain ADP-ribosylates EF2 and inhibits protein synthesis. Cells die because they cannot make new protein.
(A) The fusion of IL3 at the C terminus of the truncated diphtheria toxin. The binding domain of diphtheria toxin, located at the C terminus, is deleted and replaced by IL3. Thus, the cytotoxicity of the fusion protein is targeted to cells expressing IL3 receptors (CD123). The domain names are as follows: A, active enzyme domain; T, translocation domain; B, binding domain. The binding domain is removed and replaced by IL3 to form DT-IL3, which is called SL-401 in Frankel et al.1 (B) The pathway of binding, entry, and killing by DT-IL3. DT-IL3 binds CD123 and is internalized, and the A chain is processed proteolytically to release the A chain. The A chain translocates from acidic endosomes to the cytosol. In the cytosol, the A chain ADP-ribosylates EF2 and inhibits protein synthesis. Cells die because they cannot make new protein.
BPDCN is a rare hematological malignancy (known previously as blastic natural killer lymphoma or natural killer cell leukemia and other similar names) that commonly presents in the skin and progresses to a leukemic phase. Typically, malignant blasts are CD4+, CD56+, and CD123+.2 In a small phase 1-2 study, the authors report a high rate of complete remissions in patients diagnosed with BPDCN and receiving diphtheria toxin (DT)-IL3 as a single agent. This is a remarkable achievement. Apparently, malignant cells expressing IL3 receptors (CD123 is the α subunit of the IL3 receptor) bind and internalize the DT-IL3 fusion protein, leading to inhibition of protein synthesis and cell death (see panel B). In their paper, DT-IL3 is called SL-401
In a landmark paper, >30 years ago, Thorpe et al suggested that DT could be engineered to kill leukemia cells.3 Frankel et al fulfill that promise and produced a functional example of a DT fusion protein that demonstrates clear clinical benefit for patients with a hematological malignancy. The results of more advanced trials in the BPDCN population and treated with DT-IL3 are now eagerly awaited. Further, because these results were achieved with a single agent, future studies will undoubtedly strive to identify suitable agents to combine with DT-IL3 and improve its efficacy.
The use of protein toxins such as diphtheria toxin, Pseudomonas exotoxin, and ricin to kill malignant cells is particularly attractive because of the potency associated with the enzyme domains of these toxins. The targeting of protein toxins (antibody-toxin chimeric proteins are frequently termed “immunotoxins”) was reviewed recently by Wayne et al, especially as it relates to the treatment of leukemia.4 In sum, protein toxins are not mutagenic, not subject to common pathways of drug resistance, and can be engineered easily into fusions or conjugates with cancer-binding antibodies or cytokines. The Achilles’ heel of toxin-based proteins is their immunogenicity. When given to patients with hematologic malignancies, several cycles of treatment can sometimes be administered if there is disease-induced immunosuppression or prior chemotherapies.5 However, in this study, Frankel et al remark on the problematic situation of prior DT vaccinations that apparently prime patient antibody memory responses and limit effective treatment to 1 cycle. In light of this, it should be noted that efforts to remove epitopes from protein toxins or quell antibody responses to toxins are being pursued in both preclinical and clinical settings and may ultimately allow multiple treatment cycles with toxin-based therapeutics.6,7 Thus, the prospects for repeated administrations of toxin-based therapeutics are apparently improving.
BPDCN qualifies for targeting by DT-IL3 by virtue of expressing the IL3 receptor (CD123) on the surface of malignant cells.2 Typically, the binding of IL-3 transmits growth and survival signals to the cell interior via phosphorylation of key effectors. DT-cytokine fusions may initially (minutes to hours) generate proliferation signals, but then as the toxin gains access to the cytosol, protein synthesis will be inhibited (hours) and cells will die (days) when they cannot make new proteins. Although BPDCN is relatively rare, expression of IL3 receptors is not. Specifically, CD123 is expressed on the surface of various B-cell and myeloid malignancies and as such could be targeted by agents such as DT-IL3 but also by such agents as chimeric antigen receptor T cells or immunotoxins directed to CD123—citations to these studies are found in Frankel et al. In addition, CD123 is expressed on various nonmalignant cells, and damage to these cells must also be addressed. Here the picture is not entirely clear. The preponderance of evidence suggests that CD123 is not expressed on precursor or stem cells but rather on more mature cells such as basophils, eosinophils, macrophages, and megakaryocytes.8,9 Whether or not targeting cytotoxic agents to CD123-expressing normal human cells will cause serious adverse events in the form of cell lineage depletion remains to be determined and will likely depend on careful evaluation of patients receiving treatments such as those described by Frankel et al. For now, however, the community should rejoice in the publication of a study reporting on major patient responses in a disease that is very difficult to treat with existing agents.
Conflict-of-interest disclosure: The author declares no competing financial interests.