In this issue of Blood, Gagne et al describe a cohort of 362 patients clinically classified as having Diamond-Blackfan anemia (DBA), in which 175 (48%) were found to have mutations and deletions in ribosomal protein genes or GATA1, and 8 of the remaining patients (2.2% overall) had mitochondrial gene deletions consistent with Pearson marrow-pancreas syndrome (PS). The authors propose that all patients with presumptive DBA should be tested for mitochondrial DNA (mtDNA) deletion during their initial genetic evaluation.1
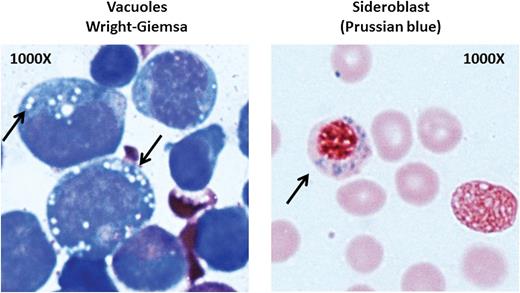
Bone marrow from a patient with PS. Left: vacuoles in myeloid precursors; right: a ringed sideroblast. See Figure 1E in the article by Gagne et al that begins on page 437.
Bone marrow from a patient with PS. Left: vacuoles in myeloid precursors; right: a ringed sideroblast. See Figure 1E in the article by Gagne et al that begins on page 437.
Sometimes one syndrome turns out to be another: for example, newborn anemia that was first thought to be DBA (MIM #105650) is instead found to be PS (MIM#557000) on genetic evaluation. Should DBA and PS be distinguished clinically first? About 25% of patients with DBA may have characteristic physical features, such as short stature, low birth weight, abnormal thumbs, cleft lip or palate, and congenital heart disease among others.2 These features are not commonly seen in PS (except low birth weight), and the patients in the Gagne article do not show a specific pattern of physical features. Although both DBA and PS present in infancy with anemia and erythroid hypoplasia, the former do not usually have neutropenia and thrombocytopenia, which were present in all of the PS patients described by Gagne et al. There is a reasonably sensitive and specific blood test for untransfused patients with DBA—red cell adenosine deaminase (ADA)—which is elevated in about 85% of patients with DBA3 ; elevated ADA was reported once in PS but the type of specimen was not clear,4 and red cell ADA has not been examined systematically in PS.
Macrocytic red cells, low reticulocyte levels, and elevated hemoglobin F are common in any type of inherited bone marrow failure syndrome and do not distinguish DBA from PS; thus, bone marrow tests may be the most informative. In DBA, the marrow shows erythroid hypoplasia but is otherwise usually normal without significant dyspoiesis. In PS, however, the marrow is usually abnormal, with global hypocellularity and vacuoles in myeloid and erythroid precursors, as were found in the PS patients in the Gagne article (see figure). Vacuolated precursors are not a feature of DBA; they may be seen as an artifact or associated with infection, particularly infection due to parvovirus B19. Their absence in early marrows in the patients studied by Gagne et al is not clearly understood, because vacuoles were seen as early as 2 weeks of age in 1 of their patients. Another feature of marrow in mitochondrial disease is ringed sideroblasts resulting from iron deposition surrounding the nucleus of erythroblasts. The diagnosis of ringed sideroblasts requires at least 5 siderotic granules per cell surrounding at least one-third of the nucleus in at least 15% of erythroblasts (see figure). In general, patients who are heavily transfused and who have an overload of iron may have sideroblasts, but they are not ringed. The differential diagnosis of ringed sideroblasts includes genetic syndromes in addition to PS such as hereditary X-linked sideroblastic anemia, thiamine-responsive megaloblastic anemia, and autosomal recessive mitochondrial myopathy with lactic acidosis. Acquired causes are the myelodysplastic syndrome subtype, which is refractory anemia with ringed sideroblasts, alcoholism, chloramphenicol, and copper deficiency. Perhaps the absence of ringed sideroblasts in very young patients with PS reflects low iron stores and thus should not be used to rule out PS.
The differential diagnosis of pure red cell aplasia anemia in infancy is very short, either DBA or transient erythroblastopenia of childhood. The physical features mentioned earlier may point toward DBA in some cases. Careful examination of the marrow for vacuoles and ringed sideroblasts may suffice to suggest PS. Pancytopenia, as well as signs of liver or renal functional disorders, and particularly lactic acidosis, should point toward PS as well.
There is no doubt that patients need to be properly classified, since those with DBA may respond to treatment with steroids, may have a remission, or may benefit from a stem cell transplant. They need genetic counseling and counseling with regard to their risk of leukemia or solid tumors.5 Family members who have the same mutated DBA gene should not be used as transplant donors, even if they are not anemic. Conversely, patients with PS have a different prognosis, with probably lower risk of malignancies, but very high risk of development of acidosis, metabolic problems, pancreatic dysfunction, and shorter life expectancy, as well as evolution to Kearns-Sayre neurologic syndrome. PS patients may have improved blood counts and become transfusion independent in childhood, but a spectrum of nonhematologic problems of mitochondrial disease may develop. The median survival in DBA is ∼40 years, although it is less than 5 years for PS patients in the literature6 and in the Gagne et al study. Molecular testing for mtDNA deletions should be used to confirm a clinical suspicion of PS, but it may not be necessary to include this test in the workup of patients who clearly have DBA, despite lack of molecular genetic evidence at this time, because ∼30% of known DBA patients do not have mutations in known DBA genes. This will change rapidly in the near future. Thus, appropriate classification of patients is critical but may be apparent from clinical and marrow morphologic data, which may then be confirmed by molecular studies, without such studies being offered to all patients with early-onset anemia.
Conflict-of-interest disclosure: The author declares no competing financial interests.