Key Points
Hodgkin lymphoma survivors who developed a second malignancy remain at high risk of developing subsequent malignancies.
Treatment options for these malignancies may be more restricted making early detection especially important to improving outcome.
Abstract
We assessed risk, localization, and timing of third malignancies in Hodgkin lymphoma (HL) survivors. In a cohort of 3122 5-year HL survivors diagnosed before the age of 51 years and treated between 1965 and 1995, we examined whether risk factors for second and third malignancies differ and whether the occurrence of a second malignancy affects the risk of subsequent malignancies, using recurrent event analyses. After a median follow-up of 22.6 years, 832 patients developed a second malignancy and 126 patients a third one. The risk of a second malignancy was 4.7-fold increased (95% confidence interval [CI], 4.4-5.1) compared with risk in the general population; the risk for a third malignancy after a second malignancy was 5.4-fold (95% CI, 4.4-6.5) increased. The 10-year cumulative incidence of any third malignancy was 13.3%. Compared with patients still free of a second malignancy, patients with a second malignancy had a higher risk of developing subsequent malignancies. This risk depended on age, with hazard ratios of 2.2, 1.6, and 1.1 for patients aged <25, 25 to 34, and 35 to 50 years at HL treatment, respectively. In HL survivors who had a second malignancy, treating physicians should be aware of the increased risk of subsequent malignancies.
Medscape Continuing Medical Education online
This activity has been planned and implemented in accordance with the Essential Areas and policies of the Accreditation Council for Continuing Medical Education through the joint providership of Medscape, LLC and the American Society of Hematology.
Medscape, LLC is accredited by the ACCME to provide continuing medical education for physicians.
Medscape, LLC designates this Journal-based CME activity for a maximum of 1.0 AMA PRA Category 1 Credit(s)™. Physicians should claim only the credit commensurate with the extent of their participation in the activity.
All other clinicians completing this activity will be issued a certificate of participation. To participate in this journal CME activity: (1) review the learning objectives and author disclosures; (2) study the education content; (3) take the post-test with a 75% minimum passing score and complete the evaluation at http://www.medscape.org/journal/blood; and (4) view/print certificate. For CME questions, see page 466.
Disclosures
Associate Editor Laurie Sehn served as an advisor or consultant for Roche/Genentech, Janssen, Lundbeck, Amgen, and Celgene; served as a speaker or a member of a speakers bureau for Roche/Genentech; and received grants for clinical research from Roche/Genentech. The authors and CME questions author Laurie Barclay, freelance writer and reviewer, Medscape, LLC, declare no competing financial interests.
Learning objectives
Describe the risks for second and third malignancies in survivors of Hodgkin lymphoma (HL), based on a cohort study.
Distinguish localization of second and third malignancies in survivors of HL.
Assess timing and prognosis of third malignancies in survivors of HL.
Release date: July 17, 2014; Expiration date: July 17, 2015
Introduction
Over the past decades, advances in treatment of Hodgkin lymphoma (HL) have led to strongly improved survival, with cure rates over 80%.1,2 Consequently, there is a growing number of HL survivors at risk for long-term treatment-related complications, such as second malignancies. Many studies have shown that the incidence of second malignancies in HL survivors is substantially increased compared with the general population.3-7 Only a few studies in childhood cancer8-11 and breast cancer12 survivors have reported on the development of third and additional malignancies due to previous cancer treatment and genetic factors. The risk of third malignancies in survivors of adult and pediatric HL has not been previously investigated.
It is likely that HL survivors who survived a second malignancy may remain at increased risk of developing subsequent malignancies as a consequence of their treatment of HL. The risk of third and additional malignancies might also be more strongly increased than the risk of a second malignancy, for example, due to increased prevalence of host susceptibility factors (eg, genetic predisposition, immunodeficiency).
In this study, we assessed the risk, localization, and timing of subsequent malignancies in HL survivors in a large Dutch cohort with complete follow-up. The quantification of these risks can be of use for optimization and individualization of surveillance protocols for HL survivors.
Patients and methods
Study population
We performed a cohort study in patients treated for HL in 7 Dutch University hospitals or cancer centers (The Netherlands Cancer Institute, Erasmus University Medical Center Rotterdam, Leiden University Medical Center, Vrije Universiteit Medical Center, Radboud University Nijmegen Medical Centre, Emma Children’s Hospital/Academic Medical Center, University Medical Center Utrecht) or in the affiliated hospitals of the population-based Eindhoven Cancer Registry. Patient selection and methods of data collection have been described in detail previously.7,13-15 In brief, patients were below age 51 years at HL diagnosis, first treated between 1965 and 1995 with radiotherapy and/or chemotherapy, who survived at least 5 years.
Data collection procedures
Data were collected on dates of birth and HL diagnosis, treatment (date of start of treatment, radiation fields, chemotherapy regimens, and number of cycles) for primary HL and relapses, and date of most recent medical information or death. Information on subsequent malignancies was collected directly from the medical records and/or obtained by contacting general practitioners and attending physicians in other hospitals (up to 2004: 94% complete follow-up16 ) and through linkage with the nationwide network and registry of histopathology and cytopathology17 and the population-based Netherlands Cancer Registry (NCR)18-20 up to 2010, resulting in a virtually complete follow-up for subsequent malignancies in our cohort. Second and third malignancies occurring in paired organs were included if there was evidence that the third malignancy was not a recurrence of the second one. Basal cell carcinomas of skin were not included in the analyses because these are not registered in the NCR. Although a noninvasive malignancy, we included ductal carcinoma in situ (DCIS) of the breast in our analyses. Patients diagnosed with a malignancy other than HL before HL diagnosis or within 5 years after HL diagnosis were excluded.
Second and third malignancies were considered synchronous when the interval between the 2 tumors was <6 months and metachronous if otherwise. All subsequent malignancies were categorized as occurring below or above the diaphragm to facilitate analyses related to radiotherapy fields, that is, we separated esophageal cancer from other gastrointestinal (GI) tract cancers.
Complete follow-up for vital status and dates of death was obtained up to November 2012 by linkage with the Dutch Central Bureau of Genealogy, which keeps computerized records of all deceased persons in The Netherlands since 1994.
Treatment
Over time, a wide variety of treatment regimens was used. Although primary treatment was usually given according to treatment protocols of the European Organization for Research and Treatment of Cancer,21-25 relapse treatment was generally not standardized. Radiotherapy techniques changed over the years, from orthovoltage therapy or cobalt 60 in the 1960s to linear accelerators from the 1970s onward. Individual blocks were used to shield normal tissues as much as possible. Patients usually received 40 Gy in fractions of 1.5 to 2.0 Gy when they received radiotherapy only and 30 to 36 Gy when they also received chemotherapy. Mantle field irradiation (including mediastinal, axillary, and neck nodes) was the most commonly applied radiation from the early 1970s to the late 1980s. Since the late 1980s, a growing number of patients received more limited radiation fields (involved field irradiation). For radiotherapy, patients were categorized into (1) no radiotherapy; (2) radiotherapy above the diaphragm; and (3) radiotherapy above and below or only below the diaphragm.
From the 1960s to the 1980s chemotherapy consisted mainly of mechlorethamine, oncovin (vincristine), procarbazine and prednisone (MOPP). In the 1980s, anthracycline-containing regimens such as MOPP/adriamycin (doxorubicin), bleomycin and vinblastine (ABV) and ABV with dacarbazine were introduced as part of primary treatment.
Statistical analysis
Second and third malignancy incidence rates in the cohort were compared with age-, sex-, and calendar period–specific cancer incidence rates in the Dutch population, accounting for person-years of observation. Cancer incidence data from the Eindhoven Cancer Registry26 up to 1988 and from the NCR19,20 from 1989 to 2009 were used as reference. The cancer incidence reference data were composed according to the International Agency for Research on Cancer rules27 for multiple primaries, according to which cancer is included only once in case a patient has 2 cancers in a paired organ or at sites with the same International Classification of Diseases code. In the accumulation of person-years of observation in the study population, time at risk for a second malignancy began 5 years after date of HL treatment and ended at date of diagnosis of the second malignancy, date of most recent medical information, or date of death, whichever came first. Time at risk for a third malignancy began at date of diagnosis of the second malignancy and ended at date of diagnosis of the third malignancy, date of most recent medical information, or date of death, whichever came first. The standardized incidence ratios (SIRs) for both second and third malignancies were calculated as the ratios of the observed and expected numbers of malignancies in the cohort. The absolute excess risks were calculated as the observed numbers of second and third malignancies in our cohort minus the expected numbers, divided by person-years at risk, multiplied by 10 000. The confidence intervals (CIs) of the SIRs were calculated using exact Poisson probabilities of observed numbers.28 P values for tests for heterogeneity or trend were calculated according to standard methods.29 Only invasive malignancies were included in our comparison with the general population because historical incidence rates for DCIS were not available in our analysis program. Thus, when a patient developed a DCIS of the breast as a second malignancy and invasive breast cancer as a third malignancy, the invasive breast cancer was included as the second malignancy. The cumulative incidence of second or third malignancies was estimated in the presence of death as a competing risk.30 Comparison of cumulative incidence curves was based on competing risk regression.31
We assessed whether HL survivors with a second malignancy were at a greater risk of developing a subsequent malignancy compared with those who did not develop a second malignancy using a conditional Cox recurrent event analysis. To evaluate whether treatment-associated risks were similar with regard to the development of second and third malignancies, we performed Cox recurrent event analysis, using a marginal approach, in which the second and third malignancy were assumed to be independent events. In this analysis, time at risk for a third malignancy was also calculated from 5 years after date of HL treatment and ended at date of diagnosis of the third malignancy, date of most recent medical information, or date of death. We performed this analysis separately for all malignancies and for solid nonbreast tumors occurring as second or third malignancies, as it has been shown that chemotherapy for HL is associated with a decreased risk of breast cancer,32,33 although it may increase the risk of lung cancer6,34 and GI tract cancer.35-37 When analyzing all malignancies combined, any effect of chemotherapy on certain malignancies would be diluted. Furthermore, in this analysis, we focused on patients who developed solid tumors, as most HL survivors who developed hematologic malignancies had a poor survival and were therefore not at risk for subsequent malignancies. In these analyses, primary HL treatment comprised all treatment (radiotherapy and/or chemotherapy) received within 5 years after HL, including relapse treatment. The occurrence of a HL relapse within these first 5 years was accounted for by inclusion of a separate relapse variable. Treatment of HL relapses received 5 years or more after HL was included using time-dependent variables. Further variables considered in these analyses were sex and age at HL treatment. To obtain variance estimators that adjust for within-subject correlation, we used robust estimation of the variance of estimated regression coefficients.38 Analyses were performed using STATA statistical software (STATA 11; Statacorp LP); a P value <.05 was considered statistically significant.
Results
The study population comprised 3122 HL survivors. Median age at initial HL treatment was 27.3 years (Table 1). Most patients received either radiotherapy alone (30.1%) or radiotherapy with chemotherapy (59.6%); 10.2% received chemotherapy only. Median follow-up time was 22.6 years and 23.5% of the patients were followed for >30 years.
Characteristics of the HL cohort and survivors with subsequent malignancies
| Characteristic . | . | . | Subsequent malignancies in HL survivors* . | |||||
|---|---|---|---|---|---|---|---|---|
| Entire cohort . | Second . | Third . | Fourth . | |||||
| No. . | % . | No. . | % . | No. . | % . | No. . | % . | |
| Total | 3122 | 100 | 832 | 100 | 126 | 100 | 16 | 100 |
| Sex | ||||||||
| Male | 1776 | 56.9 | 410 | 49.3 | 42 | 33.3 | 4 | 25.0 |
| Female | 1346 | 43.1 | 422 | 50.7 | 84 | 66.7 | 12 | 75.0 |
| Age at HL treatment, y | ||||||||
| Median | 27.3 | — | 29.2 | — | 26.9 | — | 20.1 | — |
| <25 | 1288 | 41.3 | 293 | 35.2 | 52 | 41.3 | 10 | 62.4 |
| 25-34 | 1011 | 32.4 | 270 | 32.5 | 45 | 35.7 | 3 | 18.8 |
| ≥35 | 823 | 26.3 | 269 | 32.3 | 29 | 23.0 | 3 | 18.8 |
| Treatment period HL | ||||||||
| 1965-1974 | 666 | 21.3 | 273 | 32.8 | 47 | 37.3 | 6 | 37.5 |
| 1975-1984 | 1043 | 33.4 | 329 | 39.5 | 61 | 48.4 | 9 | 56.3 |
| 1985-1995 | 1413 | 45.3 | 230 | 27.7 | 18 | 14.3 | 1 | 6.2 |
| HL treatment† | ||||||||
| RT only | 941 | 30.1 | 307 | 36.9 | 68 | 54.0 | 7 | 43.8 |
| CT only | 319 | 10.2 | 41 | 4.9 | 2 | 1.6 | 0 | 0 |
| RT + CT | 1862 | 59.6 | 484 | 58.2 | 56 | 44.4 | 9 | 56.2 |
| Follow-up after HL treatment, y | ||||||||
| Median | 22.6 | — | 24.6 | — | 30.5 | — | 32.1 | — |
| 5-9 | 308 | 9.9 | 66 | 7.9 | 2 | 1.6 | 1 | 6.2 |
| 10-19 | 869 | 27.8 | 210 | 25.3 | 16 | 12.7 | 1 | 6.2 |
| 20-29 | 1211 | 38.8 | 299 | 35.9 | 44 | 34.9 | 4 | 25.0 |
| ≥30 | 734 | 23.5 | 257 | 30.9 | 64 | 50.8 | 10 | 62.6 |
| Characteristic . | . | . | Subsequent malignancies in HL survivors* . | |||||
|---|---|---|---|---|---|---|---|---|
| Entire cohort . | Second . | Third . | Fourth . | |||||
| No. . | % . | No. . | % . | No. . | % . | No. . | % . | |
| Total | 3122 | 100 | 832 | 100 | 126 | 100 | 16 | 100 |
| Sex | ||||||||
| Male | 1776 | 56.9 | 410 | 49.3 | 42 | 33.3 | 4 | 25.0 |
| Female | 1346 | 43.1 | 422 | 50.7 | 84 | 66.7 | 12 | 75.0 |
| Age at HL treatment, y | ||||||||
| Median | 27.3 | — | 29.2 | — | 26.9 | — | 20.1 | — |
| <25 | 1288 | 41.3 | 293 | 35.2 | 52 | 41.3 | 10 | 62.4 |
| 25-34 | 1011 | 32.4 | 270 | 32.5 | 45 | 35.7 | 3 | 18.8 |
| ≥35 | 823 | 26.3 | 269 | 32.3 | 29 | 23.0 | 3 | 18.8 |
| Treatment period HL | ||||||||
| 1965-1974 | 666 | 21.3 | 273 | 32.8 | 47 | 37.3 | 6 | 37.5 |
| 1975-1984 | 1043 | 33.4 | 329 | 39.5 | 61 | 48.4 | 9 | 56.3 |
| 1985-1995 | 1413 | 45.3 | 230 | 27.7 | 18 | 14.3 | 1 | 6.2 |
| HL treatment† | ||||||||
| RT only | 941 | 30.1 | 307 | 36.9 | 68 | 54.0 | 7 | 43.8 |
| CT only | 319 | 10.2 | 41 | 4.9 | 2 | 1.6 | 0 | 0 |
| RT + CT | 1862 | 59.6 | 484 | 58.2 | 56 | 44.4 | 9 | 56.2 |
| Follow-up after HL treatment, y | ||||||||
| Median | 22.6 | — | 24.6 | — | 30.5 | — | 32.1 | — |
| 5-9 | 308 | 9.9 | 66 | 7.9 | 2 | 1.6 | 1 | 6.2 |
| 10-19 | 869 | 27.8 | 210 | 25.3 | 16 | 12.7 | 1 | 6.2 |
| 20-29 | 1211 | 38.8 | 299 | 35.9 | 44 | 34.9 | 4 | 25.0 |
| ≥30 | 734 | 23.5 | 257 | 30.9 | 64 | 50.8 | 10 | 62.6 |
CT, chemotherapy; RT, radiotherapy; y, years.
Pathological confirmation was obtained for an estimated 95% of all second malignancies and 90% of all third malignancies.
Included primary and salvage therapy. RT includes all radiotherapy fields.
During follow-up, 832 patients developed a second, 126 patients a third, and 16 a fourth primary malignancy. Median ages at diagnosis of the second and third malignancies were 50.5 years (interquartile range [IQR], 42.8-57.9) and 53.9 years (IQR, 47.0-60.8), respectively. The median interval between HL and the second malignancy was 19.4 years (IQR, 13.8-25.8) and the median interval between the second and third malignancy was 4.3 years (IQR, 1.0-10.1). The 5-year survival was 42.6% for patients who developed a second malignancy and 42.9% after a third malignancy. Two hundred ninety patients with a second malignancy (34.9% of all second malignancies) and 35 patients with a third malignancy (27.8% of all third malignancies) died in the first year following diagnosis.
Table 2 shows the frequency of second and third malignancies according to localization and sex. In women, breast cancer was the most frequently observed second (n = 184) and third (n = 46) malignancy, accounting for 43.9% of all second and 54.8% of all third malignancies. In men, lung cancer was the most frequent second (n = 103) and third (n = 11) malignancy, accounting for 25.4% of all second and 26.8% of all third malignancies.
Cancer site distribution for subsequent malignancies according to sex
| . | Subsequent malignancy . | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Men . | Women . | |||||||||||
| Second . | % . | Third . | % . | Fourth . | % . | Second . | % . | Third . | % . | Fourth . | % . | |
| Total* | 405 | 100 | 41 | 100 | 4 | 100 | 419 | 100 | 84 | 100 | 12 | 100 |
| All solid (nonbreast) | 297 | 73.3 | 37 | 90.2 | 3 | 75.0 | 184 | 43.9 | 32 | 38.2 | 5 | 41.7 |
| Lung | 103 | 25.4 | 11 | 26.8 | 0 | 0 | 37 | 8.8 | 5 | 6.0 | 0 | 0 |
| GI tract | 68 | 16.8 | 9 | 22.0 | 0 | 0 | 42 | 10.0 | 11 | 13.1 | 1 | 8.4 |
| Urogenital tract | 40 | 9.9 | 7 | 17.1 | 1 | 25.0 | 30 | 7.2 | 5 | 6.0 | 4 | 33.3 |
| Other | 86 | 21.2 | 10 | 24.3 | 2 | 50.0 | 75 | 17.9 | 11 | 13.1 | 0 | 0 |
| Breast | 2 | 0.5 | 1 | 2.4 | 0 | 0 | 184 | 43.9 | 46 | 54.8 | 7 | 58.3 |
| Leukemia and MDS | 29 | 7.2 | 2 | 5.0 | 0 | 0 | 18 | 4.3 | 0 | 0 | 0 | 0 |
| NHL | 57 | 14.1 | 1 | 2.4 | 1 | 25.0 | 22 | 5.3 | 5 | 6.0 | 0 | 0 |
| Unknown primary | 20 | 4.9 | 0 | 0 | 0 | 0 | 11 | 2.6 | 1 | 1.2 | 0 | 0 |
| . | Subsequent malignancy . | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Men . | Women . | |||||||||||
| Second . | % . | Third . | % . | Fourth . | % . | Second . | % . | Third . | % . | Fourth . | % . | |
| Total* | 405 | 100 | 41 | 100 | 4 | 100 | 419 | 100 | 84 | 100 | 12 | 100 |
| All solid (nonbreast) | 297 | 73.3 | 37 | 90.2 | 3 | 75.0 | 184 | 43.9 | 32 | 38.2 | 5 | 41.7 |
| Lung | 103 | 25.4 | 11 | 26.8 | 0 | 0 | 37 | 8.8 | 5 | 6.0 | 0 | 0 |
| GI tract | 68 | 16.8 | 9 | 22.0 | 0 | 0 | 42 | 10.0 | 11 | 13.1 | 1 | 8.4 |
| Urogenital tract | 40 | 9.9 | 7 | 17.1 | 1 | 25.0 | 30 | 7.2 | 5 | 6.0 | 4 | 33.3 |
| Other | 86 | 21.2 | 10 | 24.3 | 2 | 50.0 | 75 | 17.9 | 11 | 13.1 | 0 | 0 |
| Breast | 2 | 0.5 | 1 | 2.4 | 0 | 0 | 184 | 43.9 | 46 | 54.8 | 7 | 58.3 |
| Leukemia and MDS | 29 | 7.2 | 2 | 5.0 | 0 | 0 | 18 | 4.3 | 0 | 0 | 0 | 0 |
| NHL | 57 | 14.1 | 1 | 2.4 | 1 | 25.0 | 22 | 5.3 | 5 | 6.0 | 0 | 0 |
| Unknown primary | 20 | 4.9 | 0 | 0 | 0 | 0 | 11 | 2.6 | 1 | 1.2 | 0 | 0 |
GI, gastrointestinal (esophagus excluded); MDS, myelodysplastic syndrome; NHL, non-HL.
Hematologic malignancies (other than leukemia, MDS, and NHL) are excluded.
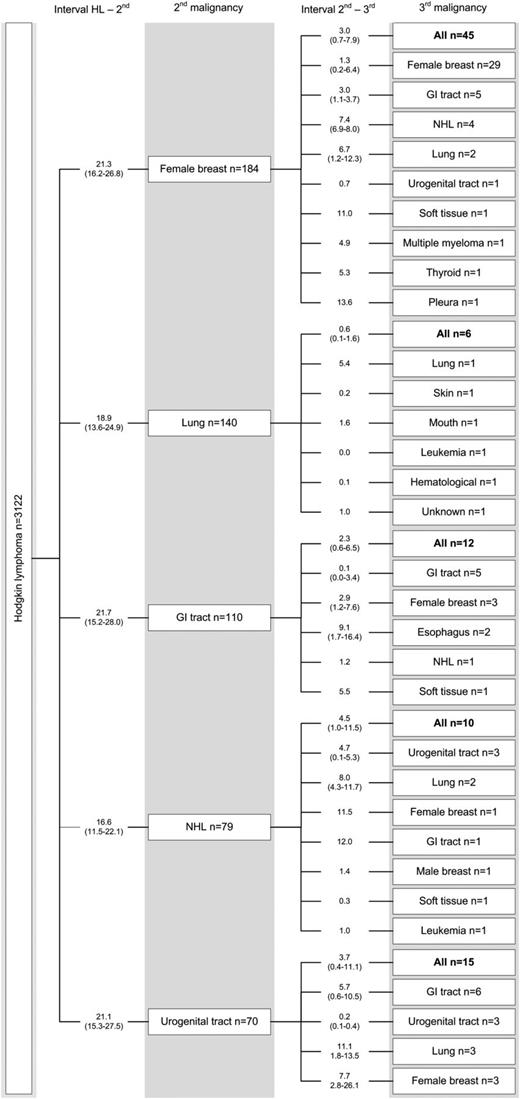
Figure 1 illustrates the pattern of occurrence of third malignancies following specific second malignancies and the time interval between malignancies. Twenty-nine of 184 women with breast cancer as their second malignancy developed another breast cancer (including 10 synchronous bilateral breast cancers), after a median interval of 1.3 years (IQR, 0.2-6.4). After a second malignancy in the GI tract (n = 110), 12 patients developed a third malignancy, of whom 5 had another GI tract cancer. Fifteen of 70 patients with urogenital cancer as their second malignancy developed a third malignancy, of which cancers of the GI tract (n = 6) were the most frequent. As expected, in patients who had a poor-prognosis second malignancy, such as lung cancer (n = 140) and esophageal cancer (n = 24) (supplemental Figure 1, available at the Blood Web site), relatively few patients developed a third malignancy (6 and 2 third malignancies, respectively). None of the 47 patients diagnosed with leukemia as a second malignancy developed a third malignancy (supplemental Figure 1); 36 patients died within 1 year after leukemia diagnosis.
Subsequent malignancies in HL survivors. Second and third malignancies occurring either as ipsilateral malignancies (with different histology) or as contralateral cancers (in paired organs) were included. “Female breast cancer” includes DCIS tumors. Twenty-four patients had a synchronous second and third malignancy (breast, n = 10; urogenital tract, n = 3; GI tract, n = 2; different sites/organ systems, n = 9). Interval in years. GI, gastrointestinal (esophagus excluded); NHL, non-HL.
Subsequent malignancies in HL survivors. Second and third malignancies occurring either as ipsilateral malignancies (with different histology) or as contralateral cancers (in paired organs) were included. “Female breast cancer” includes DCIS tumors. Twenty-four patients had a synchronous second and third malignancy (breast, n = 10; urogenital tract, n = 3; GI tract, n = 2; different sites/organ systems, n = 9). Interval in years. GI, gastrointestinal (esophagus excluded); NHL, non-HL.
For 67% of the patients, information on treatment of the second malignancy was available. Most patients were treated with surgery alone (37.0%) or chemotherapy (29.9%) for their second malignancy (Table 3). Relatively few patients received radiotherapy (14.0%) or a combination of radiotherapy and chemotherapy (7.7%).
Treatment of second malignancy in HL survivors
| . | Second malignancy . | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| All . | Female breast . | Lung . | GI tract . | NHL . | Other . | |||||||
| No. . | % . | No. . | % . | No. . | % . | No. . | % . | No. . | % . | No. . | % . | |
| Total | 832 | 184 | 140 | 110 | 79 | 319 | ||||||
| Treatment | ||||||||||||
| RT (+/− surgery) | 78 | 14.0 | 10 | 6.3 | 17 | 20.0 | 4 | 5.6 | 4 | 8.5 | 43 | 21.9 |
| CT (+/− surgery) | 167 | 29.9 | 56 | 35.0 | 29 | 34.1 | 23 | 32.4 | 28 | 59.6 | 31 | 15.8 |
| RT + CT (+/− surgery) | 43 | 7.7 | 15 | 9.3 | 9 | 10.6 | 2 | 2.8 | 12 | 25.5 | 5 | 2.6 |
| Surgery alone | 207 | 37.0 | 75 | 46.9 | 13 | 15.3 | 30 | 42.3 | 0 | 0 | 89 | 45.4 |
| Other | 64 | 11.4 | 4 | 2.5 | 17 | 20.0 | 12 | 16.9 | 3 | 6.4 | 28 | 14.3 |
| Treatment unknown | 273 | 24 | 55 | 39 | 32 | 123 | ||||||
| . | Second malignancy . | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| All . | Female breast . | Lung . | GI tract . | NHL . | Other . | |||||||
| No. . | % . | No. . | % . | No. . | % . | No. . | % . | No. . | % . | No. . | % . | |
| Total | 832 | 184 | 140 | 110 | 79 | 319 | ||||||
| Treatment | ||||||||||||
| RT (+/− surgery) | 78 | 14.0 | 10 | 6.3 | 17 | 20.0 | 4 | 5.6 | 4 | 8.5 | 43 | 21.9 |
| CT (+/− surgery) | 167 | 29.9 | 56 | 35.0 | 29 | 34.1 | 23 | 32.4 | 28 | 59.6 | 31 | 15.8 |
| RT + CT (+/− surgery) | 43 | 7.7 | 15 | 9.3 | 9 | 10.6 | 2 | 2.8 | 12 | 25.5 | 5 | 2.6 |
| Surgery alone | 207 | 37.0 | 75 | 46.9 | 13 | 15.3 | 30 | 42.3 | 0 | 0 | 89 | 45.4 |
| Other | 64 | 11.4 | 4 | 2.5 | 17 | 20.0 | 12 | 16.9 | 3 | 6.4 | 28 | 14.3 |
| Treatment unknown | 273 | 24 | 55 | 39 | 32 | 123 | ||||||
HL survivors had a 4.7-fold (95% CI, 4.4-5.1) increased SIR of developing a second malignancy compared with the general population, corresponding to 121.2 excess cases per 10 000 patient-years. A slightly higher SIR was found for a third malignancy after a second malignancy (SIR, 5.4; 95% CI, 4.4-6.5), resulting in an absolute excess risk of 309.3 per 10 000 patient-years. The SIRs for lung cancer as second and third malignancies were 6.8 (95% CI 5.7-8.1) and 6.0 (95% CI 2.5-9.8), respectively. For breast cancer as a third malignancy, the SIR was higher than for breast cancer as a second malignancy (SIR, 8.2; 95% CI, 5.6-11.4 vs SIR, 4.9; 95% CI, 4.2-5.8, respectively). For women and men, the SIRs for a third after a second malignancy were 6.2 (95% CI, 4.9-7.9) and 4.4 (95% CI, 3.1-5.9) (P value, .073), respectively. When excluding breast cancer, the SIRs were 5.2 (95% CI, 3.7-7.2) and 4.3 (95% CI, 3.0-5.8) (P value, .376), respectively.
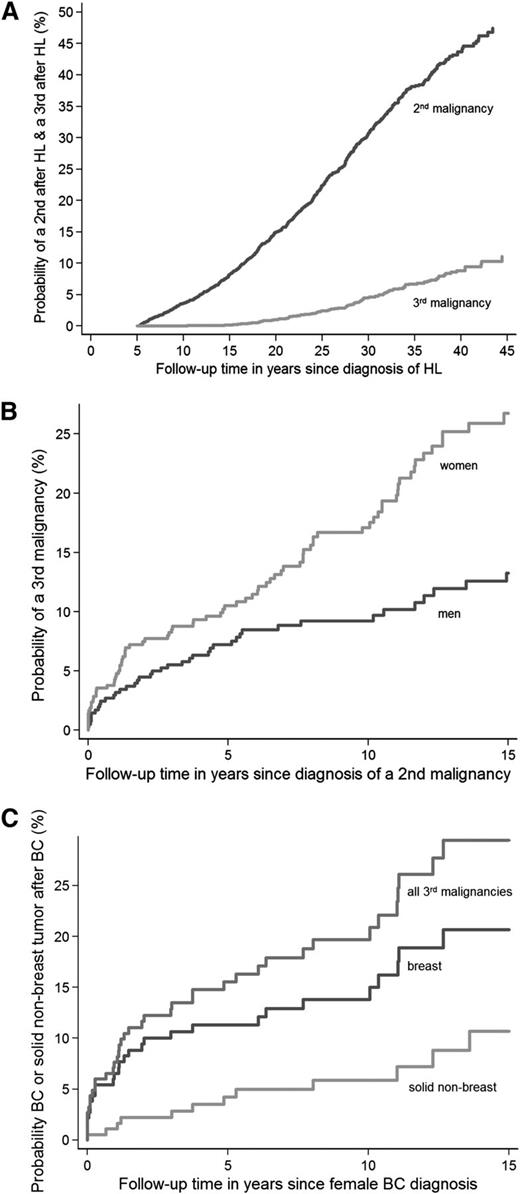
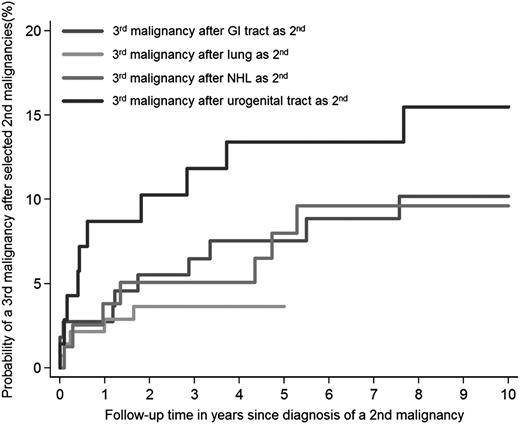
Estimated from the date of HL treatment, the 30-year cumulative incidence of developing a second malignancy was 30.7% (95% CI, 28.7-32.7) and of a third malignancy 4.6% (95% CI, 3.7-5.6) (Figure 2A). Estimated from diagnosis of the second malignancy, the 10-year cumulative incidence of any third malignancy was 13.3% (95% CI, 10.9-15.9). The 10-year cumulative incidence was higher for women (17.1%; 95% CI, 13.3-21.3) than for men (9.2%; 95% CI, 6.5-12.5; P < .001) (Figure 2B). After breast cancer, the 10-year cumulative incidence was 13.8% (95% CI, 9.0-19.6) for developing another breast cancer and 5.9% (95% CI, 2.9-10.5) for developing a solid nonbreast tumor as third malignancy (Figure 2C). The 5-year cumulative incidences of any third malignancy after cancer of the GI tract, lung cancer, non-HL, and cancer of the urogenital tract were 7.5% (95% CI, 3.5-13.6), 3.6% (95% CI, 1.4-7.8), 8.0% (95% CI, 3.3-15.5), and 13.4% (95% CI, 6.6-22.7), respectively (Figure 3).
Cumulative incidences of second and third malignancies in HL survivors. Cumulative incidence of (A) a second malignancy in HL survivors and a third malignancy in HL survivors and of (B) a third malignancy in HL survivors by sex and of (C) breast cancer or a solid nonbreast tumor after breast cancer as a second malignancy in HL survivors. BC, breast cancer.
Cumulative incidences of second and third malignancies in HL survivors. Cumulative incidence of (A) a second malignancy in HL survivors and a third malignancy in HL survivors and of (B) a third malignancy in HL survivors by sex and of (C) breast cancer or a solid nonbreast tumor after breast cancer as a second malignancy in HL survivors. BC, breast cancer.
Cumulative incidences of third malignancies following selected second malignancies. GI, gastrointestinal (esophagus excluded).
Cumulative incidences of third malignancies following selected second malignancies. GI, gastrointestinal (esophagus excluded).
Using a marginal approach, hazard ratios (HRs) for developing a second or third malignancy were found to significantly differ for sex (Pinteraction = .003), initial radiotherapy (Pinteraction = .022), and initial chemotherapy (Pinteraction = .003) (Table 4). Although men were found to have lower risks both for developing a second or a third malignancy compared with women, men had an even more strongly decreased risk (HR, 0.4) of developing a third malignancy than of developing a second malignancy (HR, 0.7). Compared with patients who did not receive initial radiotherapy, radiotherapy above and below the diaphragm was associated with a 5.2-fold increased risk of a third malignancy while increasing risk of a second malignancy 2.6-fold. Chemotherapy received for HL during the first 5 years of follow-up was not associated with risk of a second malignancy (HR, 0.9), but was associated with a significantly decreased risk of a third malignancy (HR, 0.6). The risk of both second and third malignancies increased with more advanced age at HL treatment (>35 years vs <25 years; HR, 2.4), was higher for patients who had a relapse <5 years after primary treatment, higher for patients treated with radiotherapy for a HL relapse ≥5 years after primary treatment, whereas chemotherapy for HL relapse ≥5 years after primary treatment did not affect second or third malignancy risk (HR, 0.9).
Risk factors for development of a third malignancy after a second malignancy in HL survivors
| Risk factor . | All malignancies . | Solid nonbreast tumor after solid nonbreast tumor . | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Cohort, n = 3122 . | 2nd, n = 832 . | 3rd, n = 126 . | HR . | 95% CI . | Inter action P . | Cohort, n = 3122 . | 2nd, n = 514 . | 3rd, n = 51 . | HR . | 95% CI . | Interaction P . | |
| Sex | .003 | .744 | ||||||||||
| Female | 1346 | 422 | 84 | 1.0 | 1346 | 195 | 19 | 1.0 | ||||
| Male | 1776 | 410 | 42 | 1776 | 319 | 32 | 1.3 | 1.0-1.5 | ||||
| Male (2nd malignancy) | 0.7 | 0.6-0.9 | ||||||||||
| Male (3rd malignancy) | 0.4 | 0.3-0.6 | ||||||||||
| Age at HL treatment, y | .381 | .223 | ||||||||||
| <25 | 1288 | 293 | 52 | 1.0 | 1288 | 158 | 14 | 1.0 | ||||
| 25-34 | 1011 | 270 | 45 | 1.4 | 1.2-1.7 | 1011 | 168 | 16 | 1.7 | 1.3-2.1 | ||
| 35-50 | 823 | 269 | 29 | 2.4 | 2.0-2.8 | 823 | 188 | 21 | 3.4 | 2.7-4.3 | ||
| HL treatment | ||||||||||||
| RT, <5 y* | .022 | .027 | ||||||||||
| No | 339 | 43 | 3 | 1.0 | 339 | 29 | 2 | 1.0 | ||||
| Above diaphragm | 1339 | 343 | 44 | 1339 | 210 | 14 | ||||||
| Above and below diaphragm† | 1444 | 446 | 79 | 1441 | 275 | 35 | ||||||
| Above diaphragm (2nd malignancy) | 2.1 | 1.5-2.9 | 2.2 | 1.5-3.3 | ||||||||
| Above diaphragm (3rd malignancy) | 2.8 | 0.9-9.0 | 2.0 | 0.4-8.5 | ||||||||
| Above and below diaphragm (2nd malignancy) | 2.6 | 1.9-3.7 | 2.8 | 1.9-4.1 | ||||||||
| Above and below diaphragm (3rd malignancy) | 5.2 | 1.6-16.8 | 5.3 | 1.3-22.2 | ||||||||
| CT, <5 y* | .003 | .769 | ||||||||||
| No | 1025 | 334 | 69 | 1.0 | 1025 | 193 | 21 | 1.0 | ||||
| Yes | 2096 | 498 | 57 | 2096 | 321 | 30 | 1.1 | 0.9-1.4 | ||||
| Yes (2nd malignancy) | 0.9 | 0.8-1.1 | ||||||||||
| Yes (3rd malignancy) | 0.6 | 0.4-0.8 | ||||||||||
| RT, ≥ 5 y‡ | .764 | .846 | ||||||||||
| No | 96 | 22 | 2 | 1.0 | 96 | 15 | 1 | 1.0 | ||||
| Above diaphragm | 46 | 18 | 4 | 2.4 | 1.5-3.9 | 46 | 15 | 1 | 2.9 | 1.7-4.9 | ||
| Below diaphragm | 54 | 16 | 2 | 1.6 | 0.9-2.7 | 54 | 9 | 1 | 1.6 | 0.8-3.2 | ||
| CT, ≥ 5 y‡ | .274 | .224 | ||||||||||
| No | 39 | 13 | 4 | 1.0 | 39 | 10 | 2 | 1.0 | ||||
| Yes | 157 | 43 | 4 | 0.9 | 0.8-1.1 | 157 | 29 | 1 | 1.2 | 0.7-2.0 | ||
| Relapse <5 y after HL treatment | .475 | .217 | ||||||||||
| No | 2450 | 629 | 98 | 1.0 | 2450 | 393 | 35 | 1.0 | ||||
| Yes | 672 | 203 | 28 | 1.6 | 1.3-1.9 | 672 | 121 | 16 | 1.5 | 1.1-1.9 | ||
| Risk factor . | All malignancies . | Solid nonbreast tumor after solid nonbreast tumor . | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Cohort, n = 3122 . | 2nd, n = 832 . | 3rd, n = 126 . | HR . | 95% CI . | Inter action P . | Cohort, n = 3122 . | 2nd, n = 514 . | 3rd, n = 51 . | HR . | 95% CI . | Interaction P . | |
| Sex | .003 | .744 | ||||||||||
| Female | 1346 | 422 | 84 | 1.0 | 1346 | 195 | 19 | 1.0 | ||||
| Male | 1776 | 410 | 42 | 1776 | 319 | 32 | 1.3 | 1.0-1.5 | ||||
| Male (2nd malignancy) | 0.7 | 0.6-0.9 | ||||||||||
| Male (3rd malignancy) | 0.4 | 0.3-0.6 | ||||||||||
| Age at HL treatment, y | .381 | .223 | ||||||||||
| <25 | 1288 | 293 | 52 | 1.0 | 1288 | 158 | 14 | 1.0 | ||||
| 25-34 | 1011 | 270 | 45 | 1.4 | 1.2-1.7 | 1011 | 168 | 16 | 1.7 | 1.3-2.1 | ||
| 35-50 | 823 | 269 | 29 | 2.4 | 2.0-2.8 | 823 | 188 | 21 | 3.4 | 2.7-4.3 | ||
| HL treatment | ||||||||||||
| RT, <5 y* | .022 | .027 | ||||||||||
| No | 339 | 43 | 3 | 1.0 | 339 | 29 | 2 | 1.0 | ||||
| Above diaphragm | 1339 | 343 | 44 | 1339 | 210 | 14 | ||||||
| Above and below diaphragm† | 1444 | 446 | 79 | 1441 | 275 | 35 | ||||||
| Above diaphragm (2nd malignancy) | 2.1 | 1.5-2.9 | 2.2 | 1.5-3.3 | ||||||||
| Above diaphragm (3rd malignancy) | 2.8 | 0.9-9.0 | 2.0 | 0.4-8.5 | ||||||||
| Above and below diaphragm (2nd malignancy) | 2.6 | 1.9-3.7 | 2.8 | 1.9-4.1 | ||||||||
| Above and below diaphragm (3rd malignancy) | 5.2 | 1.6-16.8 | 5.3 | 1.3-22.2 | ||||||||
| CT, <5 y* | .003 | .769 | ||||||||||
| No | 1025 | 334 | 69 | 1.0 | 1025 | 193 | 21 | 1.0 | ||||
| Yes | 2096 | 498 | 57 | 2096 | 321 | 30 | 1.1 | 0.9-1.4 | ||||
| Yes (2nd malignancy) | 0.9 | 0.8-1.1 | ||||||||||
| Yes (3rd malignancy) | 0.6 | 0.4-0.8 | ||||||||||
| RT, ≥ 5 y‡ | .764 | .846 | ||||||||||
| No | 96 | 22 | 2 | 1.0 | 96 | 15 | 1 | 1.0 | ||||
| Above diaphragm | 46 | 18 | 4 | 2.4 | 1.5-3.9 | 46 | 15 | 1 | 2.9 | 1.7-4.9 | ||
| Below diaphragm | 54 | 16 | 2 | 1.6 | 0.9-2.7 | 54 | 9 | 1 | 1.6 | 0.8-3.2 | ||
| CT, ≥ 5 y‡ | .274 | .224 | ||||||||||
| No | 39 | 13 | 4 | 1.0 | 39 | 10 | 2 | 1.0 | ||||
| Yes | 157 | 43 | 4 | 0.9 | 0.8-1.1 | 157 | 29 | 1 | 1.2 | 0.7-2.0 | ||
| Relapse <5 y after HL treatment | .475 | .217 | ||||||||||
| No | 2450 | 629 | 98 | 1.0 | 2450 | 393 | 35 | 1.0 | ||||
| Yes | 672 | 203 | 28 | 1.6 | 1.3-1.9 | 672 | 121 | 16 | 1.5 | 1.1-1.9 | ||
HRs based on Cox recurrent event analyses with marginal approach. 2nd, second malignancy; 3rd, third malignancy.
y, years.
Includes all relapse treatment within 5 years after HL (672 patients with relapse within 5 years).
Includes 316 patients treated with radiotherapy below the diaphragm only.
Treatment of relapse 5 years or more after HL, time-dependent (196 patients with relapse ≥5 years after HL).
When analysis was restricted to solid nonbreast tumors, HRs for second or third malignancies did not differ, except for the risk associated with initial radiotherapy (Pinteraction = .027). Compared with no radiotherapy, radiotherapy above and below the diaphragm was associated with a significantly higher risk of developing a third malignancy (HR, 5.3) than of a second malignancy (HR, 2.8). Male HL patients had a higher risk of developing either a second or third solid nonbreast tumor compared with female patients, as did patients who were older at HL treatment and patients who received radiotherapy for a HL relapse ≥5 years after primary treatment.
In a conditional recurrent event analysis, the risk of developing a subsequent malignancy was 1.5-fold increased (HR; 95% CI, 1.3-1.9) in patients who already developed a second malignancy compared with patients who were still free of a second malignancy. However, this risk differed according to age at HL treatment (Pinteraction = .020); the risk was 2.2-fold higher (95% CI, 1.5-3.2) for patients aged <25 years and 1.6-fold higher (95% CI, 1.1-2.3) for patients aged 25 to 34 years at HL treatment, but not increased for older patients (HR, 1.1; 95% CI, 0.7-1.7).
Discussion
To the best of our knowledge, this is the largest study evaluating the long-term risk of third malignancies in both adult and pediatric HL survivors. In a multicenter cohort of HL survivors with virtually complete follow-up, we show that patients who developed a second malignancy remain at increased risk of developing subsequent malignancies (SIR, 5.4) compared with the general population. The median age at diagnosis of a third malignancy was very young (53.9 years). The 10-year cumulative incidence of any third malignancy after a second malignancy was significantly higher among women (17.1%) than among men (9.2%). Radiotherapy was associated with a somewhat higher risk of developing a third malignancy than a second malignancy. Chemotherapy received for HL during the first 5 years of follow-up was not associated with risk of a second malignancy, but was associated with a decreased risk of a third malignancy. Compared with patients still free of a second malignancy, patients who had developed a second malignancy had a higher risk of developing a subsequent malignancy. This risk depended on age, with HRs of 2.2, 1.6, and 1.1 for patients aged <25, 25 to 34, and 35 to 50 years at HL treatment, respectively.
In interpreting our results regarding the localization of subsequent malignancies it is important to consider that survival of the second malignancy is required for the development of subsequent malignancies.39 Third malignancies were relatively common among female HL survivors who developed breast cancer as a second malignancy because breast cancer has a more favorable prognosis than, for example, lung cancer or leukemia. Men more often developed second malignancies with a poor prognosis (eg, lung cancer) compared with women and therefore had less time to develop third malignancies. But also when we took survival time after a second malignancy into account, women had a higher cumulative incidence of a third malignancy than men due to their high incidence of breast cancer. However, when comparing SIRs between men and women, accounting for background cancer incidence in the general population, this difference was not significant. When restricting analyses to nonbreast cancers, no male/female difference was observed, neither for SIRs nor for cumulative incidence.
Although the median interval between HL and the second malignancy was 19.4 years, the median interval between the second and third malignancy was only 4.3 years. The latter short interval can partly be explained by the fact that once patients have survived the “latency period” between the HL treatment-induced DNA damage and the clinical occurrence of a second malignancy, another treatment-related malignancy may also become clinically manifest, especially when these patients are reaching ages at which cancer becomes more common in the general population.
The 10-year cumulative incidence of any third malignancy after a second malignancy was 17.1% in women. This means that 1 in 6 women who developed a second malignancy can be expected to develop a third malignancy within 10 years after the second malignancy, conditional on surviving the latter. This is a high risk in view of the rather young age of our study population at the time these second malignancies were diagnosed and at the end of follow-up (median ages, 47.9 years [IQR, 40.5-55.7] and 51.7 years [IQR, 46.4-58.8], respectively). In comparison, the risk of developing a first primary malignancy for a Dutch woman between the ages of 45 and 55 years was estimated to be 5.4%.20
Among HL patients with breast cancer as a second malignancy, 64% of all subsequent malignancies again were breast cancers. This pattern also raised concern in the article on third malignancy risk in childhood cancer survivors.9 Among women who were tumor-free at 1 year from the first breast malignancy, 10% developed a subsequent breast malignancy within 10 years. In our analyses, we found a slightly higher 10-year cumulative incidence of 13.8% (95% CI 9.0-19.6) for breast cancer as a third malignancy after breast cancer as a second malignancy. This is much higher than the cumulative incidence of contralateral breast cancer among Dutch breast cancer patients in general, which was ∼4.2%.40 The risk of subsequent breast cancer might be underestimated if many women would have had a prophylactic contralateral mastectomy after breast cancer as a second malignancy. To get some insight into the extent into which prophylactic mastectomies might affect our results, we collected additional data on prophylactic mastectomies in the hospital where this procedure was most commonly offered. Prophylactic contralateral mastectomy was performed in 11 of 64 women with breast cancer as a second malignancy in this hospital. Most of these women underwent contralateral mastectomy several years after their breast cancer as a second malignancy. The proportion of overestimated person-time in our analysis was only 12.9%, rendering material underestimation of breast cancer risk unlikely.
Although chemotherapy did not seem to be associated with second malignancy risk, it was associated with a decreased risk of a third malignancy. The lack of an association with risk of a second malignancy can be, at least partly, explained by the inverse association of alkylating chemotherapy with risk of leukemia/myelodysplastic syndrome41 (chemotherapy strongly increases risk) and risk of breast cancer (chemotherapy decreases risk).32,33 As treatment-related leukemias mostly occurred within a short interval after HL treatment,41 leukemia was a frequent second but a very rare third malignancy whereas breast cancer remained a frequent third malignancy. As chemotherapy may differentially affect risk of breast cancer32,33 and risk of other solid tumors,6,34,42 we specifically examined whether HL- and treatment-related risk factors for solid nonbreast tumors occurring as second and third malignancies differed. We found that risks indeed were largely in the same direction, except for the risk associated with initial radiotherapy.
We found that patients who had developed a second malignancy had a higher risk of developing a subsequent malignancy compared with patients still free of a second malignancy. This risk decreased with higher age at HL treatment. This may suggest that survivors who developed a second malignancy and were young at HL treatment are either more genetically susceptible to develop cancer or received HL treatment at an age at which tissues were more susceptible to carcinogenesis, leading to the development of multiple treatment-induced malignancies.
One question which may arise is whether the risk of third malignancies is indeed due to treatment of HL and not due to treatment of the second malignancy. It is well-known that radiotherapy increases the risk of second malignancies after an induction period of >5 to 10 years. Because the median interval between the second and third malignancy was only 4.3 years (IQR, 1.0-10.1) and many patients were treated with surgery only for their second malignancy (partly due to previous radiation for HL), we consider it unlikely that treatment of the second malignancy played an important role in the development of third malignancies. On the other hand, surveillance after the second malignancy may have resulted in earlier or even increased diagnosis of a third malignancy.
Now that cancer survivors have an increasingly long life expectancy, it is important to develop appropriate methods to estimate risk of recurrent late complications. Very few studies conducted such risk assessments. Valid methods of analysis are especially needed as development of recurrent adverse events (third and additional malignancies) is dependent on survival of the first adverse event. Our study is the first one that used a conditional recurrent event approach to examine whether HL survivors with a second malignancy were at a greater risk of developing a subsequent malignancy, compared with those who did not develop a second malignancy.
Risks of late effects are expected to change over time because of changes in treatment. Risks of subsequent malignancies, for instance, are expected to decrease in more recently treated HL patients especially because radiation volumes and dose have been decreasing over time.43-45 However, both HL survivors and their treating physicians should be well aware of the fact that the risk of developing new primary malignancies remains increased after diagnosis of a second malignancy. Due to the previous HL treatment, treatment options for the second malignancy may already be limited. Treatment options for the third malignancy may become even more restricted, possibly influencing the prognosis dramatically. Therefore, detection of subsequent malignancies in an early stage is especially important to improve treatment possibilities and outcome.
There is an Inside Blood Commentary on this article in this issue.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
This work was supported by the Dutch Cancer Society (NKI 2004-3068, NKI 2010-4720).
Authorship
Contribution: A.M.v.E., M.S., B.M.P.A., and F.E.v.L. designed the study; P.J.L., A.D.G.K., J.P.d.B., J.M.Z., J.M.M.R., L.C.M.K., J.M.R., and M.W.J.L. contributed data; A.M.v.E. and M.S. performed statistical analysis and made figures; and A.M.v.E., M.S., P.J.L., A.D.G.K., J.P.d.B., J.M.Z., J.M.M.R., L.C.M.K., J.M.R., M.W.J.L., B.M.P.A., and F.E.v.L. contributed to manuscript writing and approved the manuscript.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Flora E. van Leeuwen, Department of Epidemiology, Plesmanlaan 121, 1066 CX Amsterdam, The Netherlands; The Netherlands Cancer Institute; e-mail: f.v.leeuwen@nki.nl.