Abstract
Autologous stem cell transplantation (ASCT) has long been considered frontline therapy for newly diagnosed myeloma patients. This Spotlight examines the role of ASCT in the era of novel drugs and argues that ASCT should continue to be considered for eligible patients. A combination of novel drugs with ASCT in a sequential treatment approach can attain long-term survival and perhaps cure a subset of patients. ASCT will likely remain an important platform to develop curative strategies in the foreseeable future.
Introduction
The introduction of doublet and triplet novel drug therapy, which are well tolerated and induce high response rates, has raised the question of whether autologous stem cell transplantation (ASCT) should be performed upfront or is best reserved for relapse.1 Some experts hold the view that myeloma can be managed in some cases with novel drugs only. This is based on the premise that myeloma can be converted to a chronic disease in selected patients.2 Several arguments can be made which support the continued integral role of ASCT in the management of myeloma.
ASCT has rendered myeloma a curable disease
Long-term follow-up studies show that ASCT can achieve profound cytoreduction and likely cure a portion of patients even before the introduction of novel drugs. Total Therapy 1 (TT1), the first tandem ASCT trial for myeloma, enrolled 231 patients; with a median follow-up of 17 years, 23 remain alive and progression-free with a plateau on the overall survival (OS) curve appearing around 14 years.3 Martinez-Lopez et al reported on 344 patients who received ASCT between 1989 and 1998 who had a median follow-up of 153 months. A plateau in OS appeared after 11 years and, with a further follow-up of 5 years, no relapses were observed.4 Other studies with long-term follow-up in excess of 10 years have also found OS rates in the order of 10% to 15% with the best outcomes in younger patients.5,6
Early intensive ASCT-based therapy may prevent clonal evolution
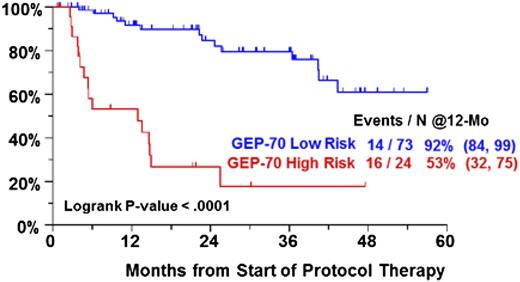
Recent genomic studies have shown that patients can acquire both linearly derived and branching clonal evolution; strategies based on controlling “sequenced therapy,” rather than eradication of myeloma, carry the risk of acquiring adverse mutational changes over time, resulting in more aggressive disease or alternatively allowing for the emergence of aggressive subclones. Repeated applications of salvage therapy can also potentially lead to shortened periods of disease control.7 Previously treated patients enrolled in our Total Therapy 6 (TT6) protocol had significantly worse gene expression profiling (GEP) defined risk factors than patients treated on Total Therapy 3 (TT3) who had received either no or minimal treatment (GEP70: 25% vs 14%, P = .025; GEP80: 54% vs 8%, P < .001). Furthermore, GEP-defined TT6 high-risk patients had a particularly dismal outcome with melphalan (MEL)–based ASCT, supporting the notion that sequential non-ASCT–based therapy may eventually allow for drug-refractory high-risk multiple myeloma (MM) to emerge (Figure 1). These findings also argue against the idea that MEL-based ASCT is not merely another line of therapy and that disease refractory to novel agents should always still be sensitive to ASCT. Conceptually, one could argue that, in younger patients, ASCT should be applied upfront to maximize clonal restriction with the objective of attaining a cure rather than to await the inevitable relapse.
PFS by GEP70 risk in TT6 for previously treated, but nontransplanted, patients.
ASCT outcomes are improved by induction with novel drugs
Incorporation of novel drugs such as proteasome inhibitors and immunomodulatory drugs in the context of ASCT during induction, consolidation, and maintenance phases has markedly upgraded responses in a number of phase 2 and phase 3 studies and substantially prolonged progression-free survival (PFS) (selected treatment schemata summarized in Table 18-15 ). Furthermore, in some of these studies adverse cytogenetic features such as t(4;14) and deletion 17p were overcome. ASCT in single or tandem fashion has been the backbone on which these studies were built and the favorable outcomes argue against the early abandonment of ASCT with long-term follow-up data lacking in nontransplant approaches using novel drugs only. In the French 2005-01 study, induction with bortezomib and dexamethasone (VD) produced higher complete response (CR)/near complete response (nCR), very good partial response (VGPR), and overall response rates compared with vincristine, adriamycin, and dexamethasone (VAD).16 These response rates remained higher post-ASCT with a trend to a better 3-year PFS in the VD arm (36 months vs 30 months, P = .064). The Spanish Myeloma Group found that induction with the triplet bortezomib, thalidomide, and dexamethasone (VTD) was superior to thalidomide and dexamethasone or conventional chemotherapy with added bortezomib.9 The CR rates in the 3 arms postinduction and post-ASCT were 35% and 46%, 14% and 24%, and 21% and 38%, respectively, which translated into superior median PFS in the VTD arm (56 months vs 36 months vs 28 months, P = .01). The Intergroupe Francophone du Myélome (IFM) also recently reported on a phase 3 trial (2007-02) comparing VD to VTD. VTD induced significantly higher VGPR rates (50% vs 36%, P = .047) but identical CR rates (14% vs 12%).8 This improvement persisted after high-dose therapy (HDT) (VGPR or better: 66% vs 54%, P = .044). The Hemato-Oncologie voor Volwassenen Nederland 65 (HOVON-65)/GMMG-HD4 trial examined the efficacy of induction with VAD followed by single or double ASCT and thalidomide maintenance with a bortezomib-based induction together with adriamycin and dexamethasone (PAD), and post-ASCT maintenance with bortezomib. (n)CR rates were superior after PAD induction (31% vs 15%; P < .001) and bortezomib maintenance (49% vs 34%; P < .001) with significantly better PFS in the PAD arm (35 months vs 28 months).11 Bortezomib during induction and maintenance abrogated the deleterious effect of del17p on both PFS and OS.17 Conversely, bortezomib may not always overcome the deleterious effect of deletion 17p in the nontransplant setting.18
Treatment schemata of selected clinical trials
| Trial . | Induction . | Transplant . | Consolidation/Other . | Maintenance . | ||
|---|---|---|---|---|---|---|
| IFM 2007-20028 | Randomization N = 199 | Arm A: VD × 4 | MEL 200 mg/m2 + ASCT | Physician discretion | Physician discretion | |
| Arm B: vTD × 4 | ||||||
| PETHEMA/GE Study9 | Randomization N = 386 | Arm A: VBMCP/VBAD/ × 4 and V × 2 | MEL 200 mg/m2 or MEL 100 mg/m2 × 2 + ASCT | None | Randomization #2 | Interferon × 3 |
| Arm B: TD ×6 | T × 3 years | |||||
| Arm C: VTD × 6 | VT × 3 years | |||||
| GIMEMA Study10 | Randomization N = 480 | Arm A: VTD × 3 | MEL 200 mg/m2 + ASCT × 2 | Arm A: VTD × 2 | D only until relapse/toxicity | |
| Arm B: TD × 3 | Arm B: TD × 2 | |||||
| Hovon-65/ GMMM-HD411 | Randomization N = 827 | Arm A: VAD × CAD | MEL 200 mg/m2 + ASCT × 2 (GMM) or MEL 200 mg/m2 + ASCT × 1 (HOVON) | None | Arm A: T × 2 years | |
| Arm B: PAD* × 3 + CAD | Arm B: V × 2 years | |||||
| (62 patients received RIC allo-SCT post-ASCT × 1 if HLA-identical sibling available) | ||||||
| Total Therapy 3A/B12 | No randomization N = 480 | VTD-PACE × 2 | MEL 200 mg/m2 + ASCT × 2 | VTD-PACE × 2 | VTD/TD × 3 years (TT3A study) VRD × 3 years (TT3B study) | |
| Total Therapy 413 | Randomization N = 345 | Standard arm: MVDT-PACE × 2 | Standard arm: MEL 200 mg/m2 + ASCT × 2 | Standard arm: VTD-PACE × 2 | VRD × 3 years | |
| “Lite” arm: MVTD-PACE × 1 | “Lite” arm: VTD-MEL 50 mg/m2 × 4 + ASCT × 2 | “Lite” arm: VTD-PACE × 1 | ||||
| Total Therapy 5 and 613 | No randomization N = 72 | MEL-10 VTD-PACE × 1 | Mel 20 mg/m2 × 4 VRD-PACE + ASCT#1 Intertherapy: MEL 5 mg/m2 × 4 VTD-PACE × 2 cycles MEL 20 mg/m2 × 4 VRD-PACE + ASCT#2 | None | VRD × 3 years | |
| MMR-V-Pl-20914 | 2 × 2 factorial Randomization N = 403 | Arms A-D: RD × 4 | Arms A-B: MEL 200 mg/m2 + ASCT × 2 | Arms A-B: no ASCT | Arms A, C: R until relapse | |
| Arms B-C: none | Arms B-C: MPR × 6 | Arms B, C: none | ||||
| CRD vs MEL 20015 | 2 × 2 factorial Randomization N = 389 | Arms A-D: RD × 4 | Arms A-B: no ASCT | Arms A-B: CRD × 6 | Arms A, C: RP until relapse | |
| Arms C-D: MEL 200 mg/m2 + ASCT × 2 | Arms C-D: none | Arms B, C: R until relapse | ||||
| Trial . | Induction . | Transplant . | Consolidation/Other . | Maintenance . | ||
|---|---|---|---|---|---|---|
| IFM 2007-20028 | Randomization N = 199 | Arm A: VD × 4 | MEL 200 mg/m2 + ASCT | Physician discretion | Physician discretion | |
| Arm B: vTD × 4 | ||||||
| PETHEMA/GE Study9 | Randomization N = 386 | Arm A: VBMCP/VBAD/ × 4 and V × 2 | MEL 200 mg/m2 or MEL 100 mg/m2 × 2 + ASCT | None | Randomization #2 | Interferon × 3 |
| Arm B: TD ×6 | T × 3 years | |||||
| Arm C: VTD × 6 | VT × 3 years | |||||
| GIMEMA Study10 | Randomization N = 480 | Arm A: VTD × 3 | MEL 200 mg/m2 + ASCT × 2 | Arm A: VTD × 2 | D only until relapse/toxicity | |
| Arm B: TD × 3 | Arm B: TD × 2 | |||||
| Hovon-65/ GMMM-HD411 | Randomization N = 827 | Arm A: VAD × CAD | MEL 200 mg/m2 + ASCT × 2 (GMM) or MEL 200 mg/m2 + ASCT × 1 (HOVON) | None | Arm A: T × 2 years | |
| Arm B: PAD* × 3 + CAD | Arm B: V × 2 years | |||||
| (62 patients received RIC allo-SCT post-ASCT × 1 if HLA-identical sibling available) | ||||||
| Total Therapy 3A/B12 | No randomization N = 480 | VTD-PACE × 2 | MEL 200 mg/m2 + ASCT × 2 | VTD-PACE × 2 | VTD/TD × 3 years (TT3A study) VRD × 3 years (TT3B study) | |
| Total Therapy 413 | Randomization N = 345 | Standard arm: MVDT-PACE × 2 | Standard arm: MEL 200 mg/m2 + ASCT × 2 | Standard arm: VTD-PACE × 2 | VRD × 3 years | |
| “Lite” arm: MVTD-PACE × 1 | “Lite” arm: VTD-MEL 50 mg/m2 × 4 + ASCT × 2 | “Lite” arm: VTD-PACE × 1 | ||||
| Total Therapy 5 and 613 | No randomization N = 72 | MEL-10 VTD-PACE × 1 | Mel 20 mg/m2 × 4 VRD-PACE + ASCT#1 Intertherapy: MEL 5 mg/m2 × 4 VTD-PACE × 2 cycles MEL 20 mg/m2 × 4 VRD-PACE + ASCT#2 | None | VRD × 3 years | |
| MMR-V-Pl-20914 | 2 × 2 factorial Randomization N = 403 | Arms A-D: RD × 4 | Arms A-B: MEL 200 mg/m2 + ASCT × 2 | Arms A-B: no ASCT | Arms A, C: R until relapse | |
| Arms B-C: none | Arms B-C: MPR × 6 | Arms B, C: none | ||||
| CRD vs MEL 20015 | 2 × 2 factorial Randomization N = 389 | Arms A-D: RD × 4 | Arms A-B: no ASCT | Arms A-B: CRD × 6 | Arms A, C: RP until relapse | |
| Arms C-D: MEL 200 mg/m2 + ASCT × 2 | Arms C-D: none | Arms B, C: R until relapse | ||||
TT4 is for newly diagnosed GEP-defined low risk MM. TT5 and TT6 employ the same treatment schedule. TT5 is for newly diagnosed GEM-defined high risk patients, while TT6 enrolls patient who had >1cycle of prior therapy, but no ASCT, regardless of GEP risk.
CRD, cyclophosphamide, lenalidomide and dexamethasone.
bortezomib.
Consolidation and maintenance with novel drugs improves PFS post-ASCT
Several studies show that consolidation and maintenance post-ASCT can further reduce tumor burden and improve outcome. The importance of post-ASCT consolidation was already reported prior to the introduction of novel drugs, by comparing the nonthalidomide arm of T2, which used consolidation with dexamethasone, cyclophosphamide, etoposide, and cisplatin (DCEP)/etoposide, dexamethasone, cytarabine, cisplatin (EDAP) and later dexamethasone, cisplatin, doxorubicin, cyclophosphamide and etoposide (DPACE), with TT1 patients who only received maintenance with interferon and dexamethasone. Five-year rates of continuous CR (45% vs 32%, P < .001) and event-free survival (EFS) (45% vs 32%, P < .001) were superior in patients receiving consolidation chemotherapy (43% vs 28%, P < .001).19 Furthermore, 4-year post-ASCT OS was significantly better in patients with metaphase cytogenetic abnormalities who received post-ASCT consolidation (76% vs 47%; P = .04). The Italian Gruppo Italiano Malattie Ematologiche dell’Adulto (GIMEMA) study group compared VTD or TD both administered pretandem and posttandem ASCT and found significantly better 5-year PFS with VTD (62% vs 49%; P = .045).10 Consolidation with VTD rather than TD significantly increased (n)CR rates (73% vs 61%; P = .02) and reduced the risk of progression by 31% (hazard ratio [HR], 0.69; P = .04). Ladetto et al studied by polymerase chain reaction (PCR) the effect of 4 cycles of VTD consolidation in 39 patients with at least VGPR status post-ASCT.20 VTD consolidation depleted tumor burden by ∼4 natural logarithms and 18% of patients achieved molecular remission (MR) with no subsequent relapses in this group with a median follow-up of 27 months. This study further underlines the importance of consolidation and emphasizes that major tumor reduction can be achieved post-ASCT.20 The Nordic Myeloma group showed in a landmark analysis that bortezomib as consolidation given for only 5 cycles produced a 7-month prolongation of PFS post-ASCT compared with patients receiving placebo (27 months vs 20 months; P = .05).21 Both the Nordic and the HOVON-65/GMMG-HD4 trials reported that bortezomib maintenance resulted in grade ≥3 peripheral neuropathy in the order of 5%.
Thalidomide has been studied as maintenance in 8 randomized studies and all studies showed improved PFS with improved duration of disease control. In 3 studies an OS benefit emerged. However, thalidomide can induce peripheral neuropathy and, in a recently reported Canadian study, maintenance with thalidomide and prednisone adversely affected quality of life.22 In the Medical Research Council Myeloma IX (MRC IX) study, patients with adverse fluorescence in situ hybridization (FISH) features seemed to fare worse in terms of OS with thalidomide maintenance especially when thalidomide induction was used, suggesting that thalidomide-resistant subclones were selected.23 This could be explained both by lack of effective salvage therapy at the time of progression or alternatively indicate that patients with adverse FISH features such as t(4;14) and deletion 17p require bortezomib.
There has been no study comparing thalidomide and lenalidomide as maintenance. However, from present data lenalidomide appears to be better tolerated post-ASCT. In the IFM 2005-02 study, lenalidomide maintenance improved the at least VGPR rates from 76% to 84% (P = .009) and improved median PFS from 23 months with placebo to 41 months with study drug (P = .009) without a discernable difference in OS.24 The Cancer and Leukemia Group B (CALGB) 1001004 study randomized patients to 10 mg of lenalidomide or placebo post-ASCT. Lenalidomide maintenance improved both median time to progression (TTP) (50 months vs 27 months, P < .001) and OS (not reached vs 73 months, P = .008).25
Achievement of negative MRD status supports the use of ASCT
Studies with novel drugs in the setting of ASCT have demonstrated that it is feasible to achieve negative minimal residual status, a finding which has previously been mainly confined to allogeneic transplant studies.26 Paiva et al reported on the minimal residual disease (MRD) status 100 days post-ASCT in patients treated on the GEM2000 protocol.27 Forty-two percent of patients were MRD negative post-ASCT by flow cytometry which had a sensitivity of 10−4. MRD-negative patients enjoyed a 5-year PFS and OS of 60% vs 22% (P < .001) and 5-year OS of 82% vs 60% (P = .002), respectively, compared with their MRD-positive counterparts. The MRC IX trial also evaluated MRD by flow cytometry with similar sensitivity and confirmed the Spanish findings with MRD-negative patients post-ASCT having significantly better median PFS (16 months vs 21 months; P < .001). In the 2 induction arms (cyclophosphamide, thalidomide, and dexamethasone and cyclophosphamide plus VAD), ASCT resulted in a 2.8- and 4.2-fold increase in MRD negativity.23 Both studies reported that patients’ MRD status was also predictive of outcome in immunofixation-negative CR patients.28 MRD positivity and adverse cytogenetic features combined to predict for poor outcome, whereas patients who achieved MRD negativity and had favorable cytogenetics fared best. However, we do not understand the significance of MRD positivity in the context of molecular subgroups, GEP-defined high risk nor therapy applied. Furthermore, myeloma is often unevenly distributed throughout the marrow and there may be sampling variation. MRD also only evaluates a random marrow aspirate and does not assess any dormant nonsecretory myelomatous cells, which can be present in focal lesions on magnetic resonance imaging (MRI), and can provide a disease reservoir for relapse. Future studies will therefore have to determine the implications of sequential MRD post-ASCT and whether MRD status impacts on type and duration of consolidation and maintenance.
Early randomized data favor ASCT and novel drugs vs novel drugs alone
Nonrandomized studies do not clearly favor early vs late ASCT. The Eastern Cooperative Oncology Group E4A03 clinical trial randomized newly diagnosed MM patients to lenalidomide with high-dose dexamethasone vs lenalidomide with low-dose dexamethasone. In a post hoc analysis landmarked after 4 cycles of lenalidomide and dexamethasone for patients under the age of 65 years, OS at 3 years was 94% with early ASCT vs 78% in patients continuing lenalidomide and dexamethasone.29 The potential benefit of ASCT is further underlined by a study by Richardson et al in which patients treated with bortezomib, lenalidomide, and dexamethasone (VRD) and early ASCT with no further therapy had equal outcome in terms of OS and PFS compared with patients treated with VRD until progression.30 A further retrospective analysis of 290 patients treated at the Mayo Clinic with immunomodulatory drug-based induction showed no difference in OS (74%) in transplant upfront delayed.31 All of the aforementioned studies were not prospective or randomized, and outcome was likely influenced by a number of confounding variables. In contrast, early results of randomized studies suggest that ASCT yields superior results compared with novel drug therapy only. Gay et al reported the results of the MMRV-PI209 trial which enrolled 402 patients ≤65 years of age who received first 4 cycles of revlimid and dexamethasone induction.14 A 2 × 2 factorial design was used to randomize to 6 cycles of consolidation with melphalan, thalidomide and prednisone vs tandem ASCT with MEL200 mg/m2 followed by second randomization to 10 mg of lenalidomide until progression or placebo.14 The 5-year PFS was significantly better in the ASCT arm 39% vs 24% (P ≤ .0001) and OS was 71% vs 62% but has not reached statistical significance. Lenalidomide maintenance was superior to placebo for 4-year PFS (37% vs 26%, P < .0001) and OS (80% vs 61%, P < .02). Palumbo used a very similar design to test efficacy of 6 cycles of CRD vs consolidation with tandem ASCT with MEL 200 mg/m2 and maintenance with lenalidomide 10 mg vs lenalidomide with prednisone 50 mg on alternating days.15 This study enrolled 389 patients <65 years who all received revlimid and dexamethasone induction. Tandem ASCT was superior to CRD and, with a median follow-up, the median PFS was not reached vs 27 months (P = .01) with no difference in OS. Maintenance with lenalidomide and prednisone prolonged median PFS by 21 months (69 months vs 48 months, P = .045) with overlapping OS curves. The nonrandomized TT3 study probably best exemplifies what is achievable by combining novel agents with ASCT in a comprehensive treatment program.12 With a median follow-up of 8.7 years, the OS and PFS are 62% and 50%, respectively. After 6 years, the survival of patients treated on TT3 approaches that of the general US population, suggesting that at least a proportion may be “operationally” cured.
A caveat in interpreting CR rates with novel drugs
The introduction of novel therapy has undoubtedly incrementally increased CR rates, which have translated in many studies into improved PFS and OS. It is important to point out that the implications of achieving CR or not should be interpreted in the context of molecular profile. It is also not understood whether all CRs are equal or may differ according to type of therapy (novel agents vs ASCT). Patients with a preceding smoldering course, monoclonal gammopathy of undetermined significance (MGUS)–like signature on GEP and those with CD2 molecular subtype myeloma do not fare worse if they do not achieve CR.32,33 High-risk MM has a fast onset of CR and similar if not higher CR rates than standard-risk myeloma.34 The highest CR rates are seen in the CD1 subtype of myeloma. However, the outcome of high risk and the CD1 type of myeloma is marred by early relapse and we have shown that sustaining CR for 3 years is of critical importance.35 Present definitions of CR also do not take into account modern imaging studies such as MRI and computed tomography–positron emission tomography (CT-PET) which have at baseline and during follow-up important prognostic implications.36-38
Should early ASCT be abandoned in favor of novel drugs alone?
The combination of carfilzomib, lenalidomide, and dexamethasone (Crd) for a planned 24 cycles with optional lenalidomide maintenance has, in a phase 1/2 study, produced impressive responses in 53 patients with newly diagnosed MM with excellent tolerance.39 Carfilzomib dosing was escalated in the phase 1 portion and dosed at 36 mg/m2. After a median of 12 cycles, 62% of patient obtained a nCR and 42% a stringently defined CR (sCR) by flow cytometry. A subset of 36 patients completed ≥8 cycles and 78% achieved (n)CR with a 61% sCR rate. A phase 2 study applied 8 cycles of Crd followed by lenalidomide maintenance in 36 patients with newly diagnosed myeloma. Sixty-three percent of patients achieved sCR/CR/nCR and a further 26% VGPR status. Among 27 nCR/sCR patients assessed by 8-color flow cytometry, all tested negative for MRD.40 These early results appear to be superior to what has been achieved with other novel drug combinations in terms of (s)CR rates. Obviously, it could be argued that combining ASCT with Crd could improve responses and potentiate outcome further. Although these early results are exciting, long-term follow-up is essential to truly appreciate the effect of therapeutic interventions. A prime example is TT2 where a survival benefit emerged in the thalidomide arm after 10 years.41 Phase 3 studies will have to determine whether these responses are durable and cure patients or whether relapses will still occur. A number of large studies are in progress which will address prospectively the role novel agents, early vs late ASCT, single vs tandem ASCT and the role of consolidation, including the IFM/DFCI2009, EMN2, and BMT CTN 0702 trials.
ASCT can cure a subpopulation of high-risk MM
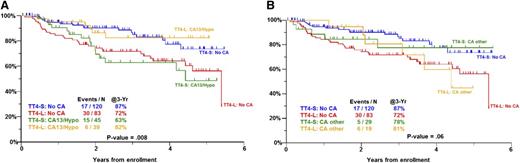
Next-generation sequencing has revealed the enormous genomic complexity of myeloma and it has been estimated that myeloma patients harbor ∼35 nonsynonymous mutations.42,43 In addition, there is considerable intraclonal heterogeneity, which is more variegated as the disease progresses. The genomic heterogeneity of myeloma may explain why the application of multiple agents with ASCT in the so-called “blunderbus” TT approach has been so successful. GEP has provided us with a number of predictive scores (eg, UAMS-70, HOVON-ECM92, IFM-15, and MR-IX-6 gene scores), which identify a subgroup of ∼15% high-risk patients. Approximately 20% of these high-risk patients will survive disease-free long-term and a cure plateau emerges much earlier at around 4 years. However, most high-risk patients are positive for MRD post-ASCT and seem destined to relapse. Clearly, ASCT and current novel agents are not adequate therapy for the majority of high-risk patients and novel therapeutic agents are sorely needed. In this group of patients, there may also be a role for optimizing the preparative regimen for ASCT by dosing melphalan based on pharmacokinetics or body weight.44,45 We and others have incorporated bortezomib in the conditioning regimen. Early results of TT4 suggest that those patients with abnormal metaphase cytogenetics who received conditioning with melphalan 50 m/m2 for 4 days together with VTD (light arm of TT4) rather than a single dose of melphalan 200 mg/m2 (standard arm of TT4) have significantly better PFS. Interestingly, the reverse applied for patient normal metaphase cytogenetics (Figure 2). These findings are supportive of the concept that bortezomib induces a “BRCA-ness state” and may sensitize more genomically unstable myeloma to DNA-damaging agents such as melphalan.13,46,47
PFS in TT4 for GEP70 low-risk myeloma by arm and metaphase cytogenetics. (A) Comparison of no CA vs hypodiploidy or del.13. (B) Comparison of no CA vs other CA. CA, metaphase cytogenetic abnormalities are present; No CA, metaphase cytogenetic abnormalities absent; Other CA, CA present, but not hypodiploidy or del.13; TT4-L, TT4 light arm; TT4-S, TT4 standard arm.
PFS in TT4 for GEP70 low-risk myeloma by arm and metaphase cytogenetics. (A) Comparison of no CA vs hypodiploidy or del.13. (B) Comparison of no CA vs other CA. CA, metaphase cytogenetic abnormalities are present; No CA, metaphase cytogenetic abnormalities absent; Other CA, CA present, but not hypodiploidy or del.13; TT4-L, TT4 light arm; TT4-S, TT4 standard arm.
Targeted therapy has already demonstrated some efficacy, but may merely select for resistant subclones, unless a given mutation drives the disease as recently has been described for KRAS/NRAS activating mutations.48 High-risk myeloma even with modern genetic analysis will likely remain a formidable opponent in years to come. Some reliance on a backbone of drugs with more pleiotropic mechanisms of actions such as immunomodulatory drugs, proteasome inhibitors, alkylators, and ASCT with added targeted therapy according to the mutational profile of an individual patient will likely be the path forward in foreseeable future.
What will the future bring?
Ultimately, the goal will have to be to further personalize therapy based on myeloma clonal profile at diagnosis, evaluation of the bone marrow microenvironment, biomarkers predicting response to drugs, and a more comprehensive response assessment embracing modern imaging. Early reduction of clonal diversity with effective therapy will likely further improve outcome and increase the likelihood of cure. It is clear that much is still to be learned and it seems likely that ASCT will remain an important option in the therapeutic armamentarium of myeloma. The results with autotransplant followed by miniallogeneic transplant have been mixed, but in 2 studies the benefit in terms of PFS and OS was noted attesting to the potential for exploiting the immune system.49,50 Presently, allogeneic transplant for myeloma should be only performed in the context of a clinical trial. However, novel immune therapeutic maneuvers such as anti-CD38 antibodies, vaccines, checkpoint blockade, and cellular therapies will likely become available and can enhance the antimyeloma response without the risk of causing serious graft-versus-host disease. It is more likely than not that these strategies will complement and not replace high-dose melphalan, which still represents one of the most active single agents in myeloma therapy today.
Acknowledgments
This work was supported by a grant from the National Cancer Institute, National Institutes of Health (grant number CA 55813).
Authorship
Contribution: F.v.R., S.G., and B.B. wrote the manuscript.
Conflict-of-interest disclosure: F.v.R. is a consultant to Janssen and has received research funding from Janssen. B.B. is a co-inventor on patents and patent applications related to use of GEP in cancer medicine. The remaining author declares no competing financial interests.
Correspondence: Frits van Rhee, Myeloma Institute for Research and Therapy, University of Arkansas for Medical Sciences, 4301 W Markham, #816, Little Rock, AR 72204; e-mail: vanrheefrits@uams.edu.