Key Points
The results of this retrospective analysis do not support intrathecal prophylaxis or radiotherapy to ECFI patients in complete remission/unconfirmed complete remission.
To define the role of radiotherapy and intrathecal prophylaxis in extralymphatic craniofacial involvement (ECFI) of aggressive B-cell lymphoma, we analyzed 11 consecutive German High-Grade Non-Hodgkin Lymphoma Study Group trials. ECFI occurred in 290/4155 (7.0%) patients (orbita, 31; paranasal sinuses, 93; main nasal cavity, 38; tongue, 27; remaining oral cavity, 99; salivary glands, 54). In a multivariable analysis adjusted for International Prognostic Index rituximab improved event-free and overall survival both in patients with and without ECFI. Three-year event-free (79% vs 79%; P = .842) and overall survival (86% vs 88%; P = .351) rates were similar in 145 patients receiving and 57 not receiving radiotherapy. Without rituximab, the 2-year cumulative rate of central nervous system (CNS) disease was increased in 205 ECFI patients compared with 2586 non-ECFI patients (4.2% vs 2.8%; P = .038), whereas this was not observed with rituximab (1.6% in 83 ECFI vs 3.4% in 1252 non-ECFI patients; P = .682). In 88 ECFI patients who received intrathecal prophylaxis with methotrexate, the 2-year rate of CNS disease was 4.2% compared with 2.3% in 191 patients who did not (P = .981). In conclusion, rituximab eliminates the increased risk for CNS disease in patients with ECFI. This retrospective analysis does not support intrathecal prophylaxis or radiotherapy to ECFI patients in complete remission/unconfirmed complete remission. These findings should be confirmed in a prospective study.
Introduction
The addition of rituximab, a monoclonal anti-CD20 antibody to cyclophosphamide/hydroxydaunorubicin/Oncovin/prednisone (CHOP) chemotherapy has improved the outcome of patients with diffuse large B-cell lymphoma (DLBCL).1,,-4 However, there are only limited data available on the prognostic impact of various sites of extralymphatic involvement in the rituximab era,5,6 and none from patients exclusively treated within prospective trials.5,7,,,,,,-14 An increased risk of central nervous system (CNS) disease has been reported for DLBCL of the paranasal sinuses, but not for other sites of extralymphatic craniofacial involvement (ECFI),9,10 and combined chemoradiotherapy has been recommended for these sites.14 We now report the results of an analysis of 11 consecutive trials of the German High-Grade Non-Hodgkin Lymphoma Study Group (DSHNHL), describe clinical characteristics, and identify factors that affect the outcome of patients with ECFI treated with and without rituximab.
Patients and methods
Studies and patients included in this analysis
Data were collected from the following 11 consecutive prospective DSHNHL trials (see supplemental Table 1 on the Blood Web site) with a median follow-up >3 years): the randomized NHL-B1,15 which compared CHOP given every 3 weeks, CHOP given every 3 weeks with the addition of etoposide (CHOEP-21), CHOP given every 2 weeks (CHOP-14), and CHOEP-14 in a 2 × 2 factorial design in young (18 to 60 years of age) patients with good prognosis (normal lactate dehydrogenase [LDH]); the NHL-B2 trial, which addressed the same question in elderly patients16 ; the High-CHOEP phase 1/2 trial, a randomized dose-escalation study of CHOEP-21 and CHOEP-14 in young good-prognosis (normal LDH) patients17 ; the High-CHOEP phase 3 study,18 which compared dose-escalated CHOEP-21 with standard CHOEP-21 in young good-prognosis (normal LDH) patients; and 2 Mega-CHOEP phase 2 studies19,20 without and 121 with rituximab, which evaluated dose-escalated CHOP plus etoposide followed by repetitive autologous stem cell transplantations in young poor-prognosis patients without and with rituximab; the MabThera International Trial (MInT) study,2 which compared CHOP-like chemotherapy regimens with and without rituximab in young (18 to 60 years of age) good-prognosis DLBCL (age-adjusted International Prognostic Index [IPI] = 0, 1; except for stage I without bulky disease) patients; the Rituximab with CHOP over Age 60 Years (RICOVER-60)3 trial, which compared 6 vs 8 cycles of CHOP-14 with and without rituximab in elderly (61 to 80 years of age) patients, the Pegfilgrastim study,22 which addressed the same question as the RICOVER-60 trial in 99 elderly patients, but where patients had an additional randomization into pegfilgrastim given on day 2 vs day 4; and the Conventional Chemo Vs HD Chemo Followed by Auto SCT in Younger Patients With Aggressive Non-Hodgkin's Lymphoma (Mega-CHOEP) phase 3 study,23 which compared 8 cycles of CHOEP with added rituximab every 2 weeks with 4 cycles of maximally dose-escalated Mega-CHOEP with added rituximab requiring 3 autologous stem cell supports in young poor-prognosis patients. In total, 4155 patients were included in this study. For details, refer to references. All ECFI sites were included in this study. Patients with involvement of the Waldeyer ring and tonsils were excluded, because the Waldeyer ring is not an extralymphatic tissue and no radiotherapy or intrathecal prophylaxis was recommended for these cases. Patients with aggressive CD20+ B-cell lymphoma were eligible for the respective trials and included in this retrospective analysis. The patients’ characteristics are shown in Table 1. The clinical trials were approved by the ethical committees of all participating institutions and were conducted in accordance with the Declaration of Helsinki.
Characteristics of patients in this study
| . | With craniofacial involvement n = 290 . | Without craniofacial involvement n = 3865 . | P value . |
|---|---|---|---|
| Sex | .008 | ||
| M | 184 (63.4%) | 2141 (55.4%) | |
| F | 106 (36.6%) | 1724 (44.6%) | |
| Age median (range) | 64 (18; 79) | 58 (18; 80) | <.001 |
| Age >60 | 169 (58.3%) | 1737 (44.9%) | <.001 |
| ECOG >1 | 9 (3.1%) | 485 (12.6%) | <.001 |
| Stage III/IV | 88 (30.3%) | 1773 (45.9%) | <.001 |
| LDH > UNV | 52 (17.9%) | 1688 (43.7%) | <.001 |
| Extralymphatic involvement >1* | 102 (35.2%) | 664 (17.2%) | <.001 |
| Bulk ≥7.5 cm | 46 (15.9%) | 1695 (43.9%) | <.001 |
| IPI = 0, 1* | 173 (59.7%) | 2057 (53.3%) | |
| IPI = 2 | 52 (17.9%) | 814 (21.1%) | .087 |
| IPI = 3 | 46 (15.9%) | 606 (15.7%) | |
| IPI = 4, 5 | 19 (6.6%) | 385 (10.0%) | |
| Lymphoblastic precursor B cell* | 0 (0.0%) | 6 (0.2%) | |
| Diffuse large B cell* | 235 (81.0%) | 3152 (81.6%) | |
| Centroblastic | 150 (51.7%) | 1669 (43.2%) | |
| Immunoblastic | 20 (6.9%) | 215 (5.6%) | |
| Plasmablastic | 3 (1.0%) | 13 (0.3%) | |
| Anaplastic large cell | 1 (0.3%) | 91 (2.4%) | |
| T-cell–rich B-cell lymphoma | 1 (0.3%) | 102 (2.6%) | |
| Mediastinal B cell | 0 (0.0%) | 219 (5.7%) | |
| DLBCL, NOS | 60 (20.7%) | 843 (21.8%) | |
| Primary effusion lymphoma* | 0 (0.0%) | 1 (<0.1%) | |
| Follicular lymphoma grade IIIb | 9 (3.1%) | 182 (4.7%) | |
| Follicular lymphoma grade III + DLBCL* | 8 (2.8%) | 143 (3.7%) | |
| Burkitt lymphoma* | 4 (1.4%) | 19 (0.5%) | |
| Burkitt-like* | 7 (2.4%) | 44 (1.1%) | |
| Mantle cell lymphoma (blastic)* | 4 (1.4%) | 38 (1.0%) | |
| Aggressive marginal zone lymphoma* | 6 (2.1%) | 36 (0.9%) | |
| NOS* | 12 (4.1%) | 118 (3.1%) | |
| B cell (unclassified, technically insufficient)* | 5 (1.7%) | 126 (3.3%) |
| . | With craniofacial involvement n = 290 . | Without craniofacial involvement n = 3865 . | P value . |
|---|---|---|---|
| Sex | .008 | ||
| M | 184 (63.4%) | 2141 (55.4%) | |
| F | 106 (36.6%) | 1724 (44.6%) | |
| Age median (range) | 64 (18; 79) | 58 (18; 80) | <.001 |
| Age >60 | 169 (58.3%) | 1737 (44.9%) | <.001 |
| ECOG >1 | 9 (3.1%) | 485 (12.6%) | <.001 |
| Stage III/IV | 88 (30.3%) | 1773 (45.9%) | <.001 |
| LDH > UNV | 52 (17.9%) | 1688 (43.7%) | <.001 |
| Extralymphatic involvement >1* | 102 (35.2%) | 664 (17.2%) | <.001 |
| Bulk ≥7.5 cm | 46 (15.9%) | 1695 (43.9%) | <.001 |
| IPI = 0, 1* | 173 (59.7%) | 2057 (53.3%) | |
| IPI = 2 | 52 (17.9%) | 814 (21.1%) | .087 |
| IPI = 3 | 46 (15.9%) | 606 (15.7%) | |
| IPI = 4, 5 | 19 (6.6%) | 385 (10.0%) | |
| Lymphoblastic precursor B cell* | 0 (0.0%) | 6 (0.2%) | |
| Diffuse large B cell* | 235 (81.0%) | 3152 (81.6%) | |
| Centroblastic | 150 (51.7%) | 1669 (43.2%) | |
| Immunoblastic | 20 (6.9%) | 215 (5.6%) | |
| Plasmablastic | 3 (1.0%) | 13 (0.3%) | |
| Anaplastic large cell | 1 (0.3%) | 91 (2.4%) | |
| T-cell–rich B-cell lymphoma | 1 (0.3%) | 102 (2.6%) | |
| Mediastinal B cell | 0 (0.0%) | 219 (5.7%) | |
| DLBCL, NOS | 60 (20.7%) | 843 (21.8%) | |
| Primary effusion lymphoma* | 0 (0.0%) | 1 (<0.1%) | |
| Follicular lymphoma grade IIIb | 9 (3.1%) | 182 (4.7%) | |
| Follicular lymphoma grade III + DLBCL* | 8 (2.8%) | 143 (3.7%) | |
| Burkitt lymphoma* | 4 (1.4%) | 19 (0.5%) | |
| Burkitt-like* | 7 (2.4%) | 44 (1.1%) | |
| Mantle cell lymphoma (blastic)* | 4 (1.4%) | 38 (1.0%) | |
| Aggressive marginal zone lymphoma* | 6 (2.1%) | 36 (0.9%) | |
| NOS* | 12 (4.1%) | 118 (3.1%) | |
| B cell (unclassified, technically insufficient)* | 5 (1.7%) | 126 (3.3%) |
ECOG, Eastern Cooperative Oncology Group; NOS, not otherwise specified; UNV, upper normal value.
According to reference pathology.
Radiotherapy
Except for the Mega-CHOEP phase 2 trials, additive radiotherapy to extralymphatic sites was recommended (but not mandatory). The dose of radiotherapy was 36 Gy with fractions of 1.8 to 2.0 Gy per day for all patients, except for 19 patients who were treated in the MInT study and received 30 to 47.5 (median 37.8) Gy.
CNS prophylaxis
CNS prophylaxis with intrathecal methotrexate (MTX; 15 mg) administered on days 1 and 5 of the first and second chemotherapy cycles was recommended for all ECFI patients.
Statistical analysis
Event-free survival (EFS)—defined as the time from randomization or start of therapy to disease progression, initiation of salvage therapy, additional (unplanned) treatment, relapse, or death—was the primary end point in all of these studies; overall survival (OS) was a secondary end point and was calculated as time from randomization to death from any cause. EFS and OS were estimated according to the Kaplan-Meier method. The estimations at 3 years were calculated with 95% confidence intervals (CI). Multivariable analyses were performed using the Cox regression model. Time to CNS disease was defined as time from randomization to disease progression or relapse in the CNS. Two-year rates of cumulative risk for CNS are presented using the cumulative incidence method. All tests for significance were at the 5% significance level. Statistical analyses were performed with SPSS (version 19).
Results
Clinical presentation
The characteristics of the patients are shown in Table 1. Roughly 10% had aggressive B-cell lymphomas other than DLBCL according to the reference pathology. A sensitivity analysis restricted to the 3387 patients with DLBCL (of whom 235 had ECFI and 3152 did not) yielded results identical to those obtained with the entire study population (data not shown). Of the 4155 patients with aggressive B-cell lymphomas, 290 (7.0%) had ECFI affecting the orbita (n = 31), paranasal sinuses (n = 93), main nasal cavity (n = 38), tongue (n = 27), the remaining oral cavity (n = 99), and salivary and parotid glands (n = 54; supplemental Table 2). ECFI was associated with male sex, normal pretreatment LDH, age >60 years, a good performance status, >1 site of extralymphatic involvement, limited stages I and II according to Ann Arbor, and nonbulky disease (Table 1).
Outcome of patients with ECFI
Roughly two-thirds of the 4155 patients (2814, or 67.7%) were treated without and one-third (1341, or 32.3%) with rituximab. Of the 290 patients with ECFI, 207 (71.4%) were treated without and 83 (28.6%) with rituximab. ECFI was similarly frequent in patients receiving (83/1341 or 6.2%) and not receiving rituximab (207/2814 or 7.4%). Median time of observation was 41 months.
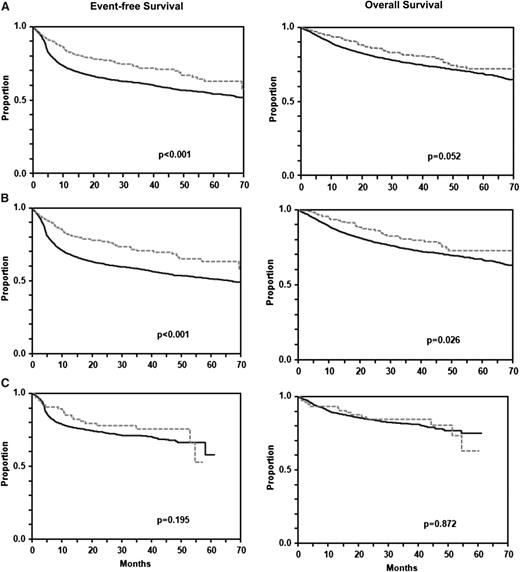
Three-year EFS (61% [95% CI 60-63] vs 72% [95% CI 66-77]; P < .001) was significantly and 3-year OS was almost significantly better (76% [95% CI 75-77] vs 82% [95% CI 77-86]; P = .052) for 290 patients with ECFI than for 3865 patients without ECFI (Figure 1A). A similar picture emerged when patients treated without rituximab (Figure 1B) were analyzed separately (3-year EFS 58% [95% CI 56-60] vs 71% [95% CI 64-77], P < .001; 3-year OS 74% [95% CI 72-75] vs 80% [95% CI 75-86]; P = .026). In contrast, in patients treated with rituximab, the differences were smaller (Figure 1C): 3-year EFS: 68% [95% CI 65-71] vs 75% [95% CI 65-85], P = .195; 3-year OS 82% [95% CI 79-84] vs 85% [95% CI 76-93], P = .872.
EFS and OS of patients with and without extralymphatic craniofacial involvement. For the entire population (A), EFS (P < .001; left) was significantly and OS was almost significantly (P = .052; right) better for 290 patients with than for 3865 patients without craniofacial involvement. A similar picture emerged from the analysis of patients treated without rituximab (B: 207 patients with and 2607 patients without craniofacial involvement), whereas in patients treated with rituximab (C: 83 patients with and 1258 patients without craniofacial involvement), the prognostic advantage of patients with extralymphatic craniofacial involvement was not observed anymore. Gray dashed curves represent patients with and black curves represent patients without extranodal craniofacial involvement.
EFS and OS of patients with and without extralymphatic craniofacial involvement. For the entire population (A), EFS (P < .001; left) was significantly and OS was almost significantly (P = .052; right) better for 290 patients with than for 3865 patients without craniofacial involvement. A similar picture emerged from the analysis of patients treated without rituximab (B: 207 patients with and 2607 patients without craniofacial involvement), whereas in patients treated with rituximab (C: 83 patients with and 1258 patients without craniofacial involvement), the prognostic advantage of patients with extralymphatic craniofacial involvement was not observed anymore. Gray dashed curves represent patients with and black curves represent patients without extranodal craniofacial involvement.
In a multivariable analysis adjusted for the IPI risk factors (Table 2), age >60 years, elevated LDH, advanced stages III/IV, poor Eastern Cooperative Oncology Group performance status (>1) were confirmed as significant prognosticators for EFS and OS. ECFI patients without rituximab had an EFS hazard ratio (HR) of 0.7 (P = .010) and of 0.9 (P = .590) with rituximab. A similar picture emerged when only the patients from MInT and RICOVER (in which patients were randomized to treatment with and without rituximab) were analyzed: ECFI patients had an HR of 0.7 (P = .115) without and of 0.9 (P = .756) with rituximab. The differences with respect to OS were smaller, and HR for ECFI was similar for patients without rituximab (HR 0.9, P = .334 for all patients; HR = 1.0, P = .924 for patients of the MInT and RICOVER trials) and with rituximab (1.1, P = .725 for all patients and 1.1, P = .687 for patients treated in the MInT and RICOVER trials). The HR of ECFI was nearly identical if bulky disease and sex were added to the Cox model (supplemental Table 3).
Multivariable analysis of craniofacial involvement
| . | All patients with and without rituximab n = 4155* . | All patients without rituximab n = 2814* . | All patients with rituximab n = 1341* . | MInT and RICOVER patients without rituximab n = 1022† . | MInT und RICOVER patients with rituximab n = 1023† . | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| . | HR . | P value . | 95% CI . | HR . | P value . | 95% CI . | HR . | P value . | 95% CI . | HR . | P value . | 95% CI . | HR . | P value . | 95% CI . |
| EFS | |||||||||||||||
| LDH > UNV | 1.4 | <.001 | 1.3-1.6 | 1.5 | <.001 | 1.3-1.7 | 1.7 | <.001 | 1.4-2.1 | 1.5 | <.001 | 1.3-1.9 | 1.6 | <.001 | 1.2-2.0 |
| Stage III/IV | 1.6 | <.001 | 1.5-1.8 | 1.7 | <.001 | 1.5-1.9 | 1.6 | <.001 | 1.3-2.1 | 1.5 | <.001 | 1.2-1.9 | 1.6 | .001 | 1.2-2.0 |
| ECOG >1 | 1.5 | <.001 | 1.3-1.7 | 1.5 | <.001 | 1.2-1.7 | 1.6 | <.001 | 1.2-2.0 | 1.8 | <.001 | 1.3-2.3 | 1.7 | .001 | 1.2-2.4 |
| Extralymphatic involvement >1 | 1.2 | .026 | 1.0-1.3 | 1.1 | .108 | 1.0-1.3 | 1.2 | .186 | 0.9-1.5 | 1.4 | .008 | 1.1-1.8 | 1.1 | .666 | 0.8-1.5 |
| Age >60 y | 1.4 | <.001 | 1.3-1.6 | 1.5 | <.001 | 1.3-1.7 | 1.3 | .007 | 1.1-1.6 | 1.0 | .991 | 0.8-1.2 | 1.3 | .039 | 1.0-1.8 |
| Craniofacial involvement | 0.7 | .008 | 0.6-0.9 | 0.7 | .010 | 0.5-0.9 | 0.9 | .590 | 0.6-1.4 | 0.7 | .115 | 0.4-1.1 | 0.9 | .756 | 0.6-1.5 |
| OS | |||||||||||||||
| LDH > UNV | 1.8 | <.001 | 1.6-2.1 | 1.9 | <.001 | 1.7-2.3 | 2.3 | <.001 | 1.7-3.1 | 2.0 | <.001 | 1.5-2.7 | 2.1 | <.001 | 1.5-3.0 |
| Stage III/IV | 1.6 | <.001 | 1.4-1.8 | 1.7 | <.001 | 1.4-2.0 | 1.6 | .003 | 1.2-2.1 | 1.3 | .055 | 1.0-1.8 | 1.4 | .066 | 1.0-2.0 |
| ECOG >1 | 1.7 | <.001 | 1.5-2.0 | 1.8 | <.001 | 1.5-2.1 | 1.7 | .001 | 1.3-2.3 | 1.9 | <.001 | 1.3-2.6 | 1.9 | .001 | 1.3-2.7 |
| Extralymphatic involvement >1 | 1.2 | .041 | 1.0-1.4 | 1.2 | .121 | 1.0-1.4 | 1.2 | .227 | 0.9-1.6 | 1.8 | .001 | 1.3-2.4 | 1.2 | .478 | 0.8-1.7 |
| Age >60 y | 2.2 | <.001 | 2.0-2.6 | 2.0 | <.001 | 1.7-2.4 | 2.5 | <.001 | 1.9-3.3 | 1.6 | .003 | 1.2-2.2 | 2.8 | <.001 | 1.8-4.3 |
| Craniofacial involvement | 0.9 | .374 | 0.7-1.2 | 0.9 | .334 | 0.6-1.2 | 1.1 | .725 | 0.6-1.9 | 1.0 | .924 | 0.6-1.8 | 1.1 | .687 | 0.6-2.0 |
| . | All patients with and without rituximab n = 4155* . | All patients without rituximab n = 2814* . | All patients with rituximab n = 1341* . | MInT and RICOVER patients without rituximab n = 1022† . | MInT und RICOVER patients with rituximab n = 1023† . | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| . | HR . | P value . | 95% CI . | HR . | P value . | 95% CI . | HR . | P value . | 95% CI . | HR . | P value . | 95% CI . | HR . | P value . | 95% CI . |
| EFS | |||||||||||||||
| LDH > UNV | 1.4 | <.001 | 1.3-1.6 | 1.5 | <.001 | 1.3-1.7 | 1.7 | <.001 | 1.4-2.1 | 1.5 | <.001 | 1.3-1.9 | 1.6 | <.001 | 1.2-2.0 |
| Stage III/IV | 1.6 | <.001 | 1.5-1.8 | 1.7 | <.001 | 1.5-1.9 | 1.6 | <.001 | 1.3-2.1 | 1.5 | <.001 | 1.2-1.9 | 1.6 | .001 | 1.2-2.0 |
| ECOG >1 | 1.5 | <.001 | 1.3-1.7 | 1.5 | <.001 | 1.2-1.7 | 1.6 | <.001 | 1.2-2.0 | 1.8 | <.001 | 1.3-2.3 | 1.7 | .001 | 1.2-2.4 |
| Extralymphatic involvement >1 | 1.2 | .026 | 1.0-1.3 | 1.1 | .108 | 1.0-1.3 | 1.2 | .186 | 0.9-1.5 | 1.4 | .008 | 1.1-1.8 | 1.1 | .666 | 0.8-1.5 |
| Age >60 y | 1.4 | <.001 | 1.3-1.6 | 1.5 | <.001 | 1.3-1.7 | 1.3 | .007 | 1.1-1.6 | 1.0 | .991 | 0.8-1.2 | 1.3 | .039 | 1.0-1.8 |
| Craniofacial involvement | 0.7 | .008 | 0.6-0.9 | 0.7 | .010 | 0.5-0.9 | 0.9 | .590 | 0.6-1.4 | 0.7 | .115 | 0.4-1.1 | 0.9 | .756 | 0.6-1.5 |
| OS | |||||||||||||||
| LDH > UNV | 1.8 | <.001 | 1.6-2.1 | 1.9 | <.001 | 1.7-2.3 | 2.3 | <.001 | 1.7-3.1 | 2.0 | <.001 | 1.5-2.7 | 2.1 | <.001 | 1.5-3.0 |
| Stage III/IV | 1.6 | <.001 | 1.4-1.8 | 1.7 | <.001 | 1.4-2.0 | 1.6 | .003 | 1.2-2.1 | 1.3 | .055 | 1.0-1.8 | 1.4 | .066 | 1.0-2.0 |
| ECOG >1 | 1.7 | <.001 | 1.5-2.0 | 1.8 | <.001 | 1.5-2.1 | 1.7 | .001 | 1.3-2.3 | 1.9 | <.001 | 1.3-2.6 | 1.9 | .001 | 1.3-2.7 |
| Extralymphatic involvement >1 | 1.2 | .041 | 1.0-1.4 | 1.2 | .121 | 1.0-1.4 | 1.2 | .227 | 0.9-1.6 | 1.8 | .001 | 1.3-2.4 | 1.2 | .478 | 0.8-1.7 |
| Age >60 y | 2.2 | <.001 | 2.0-2.6 | 2.0 | <.001 | 1.7-2.4 | 2.5 | <.001 | 1.9-3.3 | 1.6 | .003 | 1.2-2.2 | 2.8 | <.001 | 1.8-4.3 |
| Craniofacial involvement | 0.9 | .374 | 0.7-1.2 | 0.9 | .334 | 0.6-1.2 | 1.1 | .725 | 0.6-1.9 | 1.0 | .924 | 0.6-1.8 | 1.1 | .687 | 0.6-2.0 |
According to reference pathology.
Intention to treat.
In a multivariable analysis adjusted for IPI risk factors, the addition of rituximab improved EFS and OS in patients with and without ECFI: rituximab reduced HREFS and HROS to 0.6 (P < .001) in patients without ECFI, and to HR = 0.6 and HR = 0.5 for EFS and OS, respectively, in patients with ECFI. Because of the smaller number of patients, however, these HR reductions were not significant in patients with ECFI (PEFS = 0.153; POS = 0.118; supplemental Table 4).
The role of radiotherapy
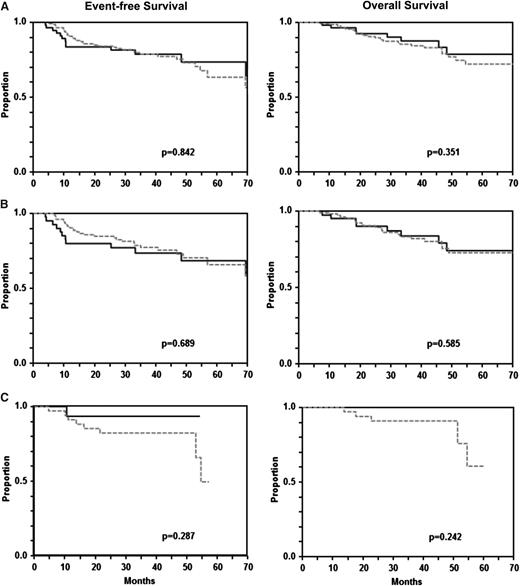
With the exception of the Mega-CHOEP phase 2 trials,19,-21 in which no radiotherapy was given, radiotherapy to extralymphatic lymphoma sites was recommended (but not mandatory) in all other trials included in this study. However, some institutions did not follow this recommendation and retained their institutional policy of not irradiating ECFI sites, leaving a considerable proportion of patients with ECFI without radiotherapy, enabling us to study the role of radiotherapy to ECFI sites. This had to be restricted to 202 ECFI patients who achieved a complete remission (CR), unconfirmed CR (CRu,) or partial remission (PR) after (immuno-)chemotherapy, because patients achieving less than a PR were regarded as treatment failures, went off protocol, and received salvage chemotherapy. Figure 2 shows the EFS and OS of these 202 patients. Patients receiving radiotherapy or not were well-balanced (supplemental Table 5). The 145 patients with radiotherapy to ECFI sites had a nearly identical EFS (79% [95% CI 67-90%] vs 79% [95% CI 72-86%]: P = .842) and OS (88% [95% CI 78-97] vs 86% [95% CI 80-92%]; P = .351) when compared with the 57 patients who did not receive radiotherapy. The results where similar when ECFI patients treated and without rituximab were analyzed separately (Figure 2): 3-year EFS with and without radiotherapy was 74% (95% CI 59-88) vs 77% (95% CI 69-86; P = .689); 3-year OS was 84% (95% CI 71-96) vs 84% (95% CI 76-91; P = .585) for patients without rituximab; for patients with rituximab, 3-year EFS was 93% (95% CI 81-100) without vs 82% (95% CI 69-94) with radiotherapy (P = .287); 3-year OS was 100% without vs 92% (95% CI 83-100; P = .242) with radiotherapy. A multivariable analysis adjusted for bulky disease and the IPI risk factors age, LDH, advanced stage, and >1 extralymphatic site of involvement (supplemental Table 6) confirmed that radiotherapy had no impact on EFS (HR 1.0 [95% CI 0.5-2.0]; P = .919) or OS (HR 1.5 [95% CI 0.7-3.3]; P = .358). This also held true when CR vs CRu/PR was added to the model. A model including patients in PR vs CR/CRu after (immuno-)chemotherapy only (which was less stable, because only 1 patient in PR did not receive radiotherapy to ECFI sites) also showed no positive effect of radiotherapy for EFS (HR = 1.0; P = .982; data not shown).
EFS and OS of patients with extralymphatic craniofacial involvement treated without and with radiotherapy. There was no difference in EFS (A, left) and OS (B, right) between 145 patients with craniofacial involvement who received additive radiotherapy and 57 patients who did not. A similar picture emerged from the analysis of patients treated without rituximab (B: 107 patients who received radiotherapy, 41 patients who did not) and with rituximab (C: 38 patients who received radiotherapy, 16 patients who did not). Gray dashed curves represent patients who received and black curves represent patients who did not receive radiotherapy.
EFS and OS of patients with extralymphatic craniofacial involvement treated without and with radiotherapy. There was no difference in EFS (A, left) and OS (B, right) between 145 patients with craniofacial involvement who received additive radiotherapy and 57 patients who did not. A similar picture emerged from the analysis of patients treated without rituximab (B: 107 patients who received radiotherapy, 41 patients who did not) and with rituximab (C: 38 patients who received radiotherapy, 16 patients who did not). Gray dashed curves represent patients who received and black curves represent patients who did not receive radiotherapy.
Relapse pattern
Thirty-eight patients with ECFI relapsed, of which 9 were treated with rituximab and 29 without (supplemental Table 7). Of note, only 1 of these 38 patients had a local relapse; all other patients relapsed at distant sites. Of the 29 patients treated without rituximab, 19 (66%) had localized and 9 (31%) had disseminated disease; the stage was unknown in 1 patient (3%). One patient with localized disease relapsed only locally (even though he had received local radiotherapy) and 18 patients relapsed at distant sites, irrespective of whether they had received local radiotherapy (12 patients) or not (6 patients).
Of the 9 patients who had received rituximab and relapsed, 2 (22%) had localized disease and 7 (78%) had advanced disease. All rituximab-treated ECFI patients relapsed at distant sites, irrespective of whether they had received radiotherapy (n = 5) or not (n = 4). The 2 patients with localized disease had no bulky disease and had received radiotherapy to EFCI; they relapsed with CNS and disseminated disease, respectively. Seven patients with ECFI had advanced disease, 3 received radiotherapy and 4 did not, including the only 2 patients with ECFI and bulky disease who relapsed. All of them relapsed outside the ECFI site.
CNS disease
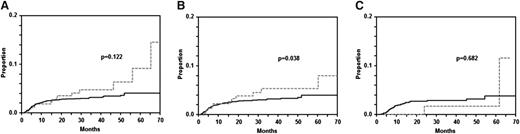
Twenty-nine patients with primary CNS involvement (2 patients with ECFI und 27 without) at diagnosis were excluded in this analysis. The characteristics of the other ECFI patients developing CNS disease is shown in supplemental Table 8. The 2-year rates of cumulative risk for CNS disease in 288 patients with ECFI was 3.5% [95% CI: 1.1-5.9] compared with 3.0% [95% CI: 2.4-3.6] in 3838 patients without ECFI (P = .122; Figure 3). In patients treated without rituximab, the 2-year rate of cumulative risk of CNS disease was significantly higher in 205 ECFI patients than in 2586 patients without ECFI (4.2% [95% CI: 1.3-7.1] vs 2.8% [95% CI: 2.2-3.4]; P = .038; Figure 3). The respective figures for patients treated with rituximab showed a reversed trend for CNS disease: the 2-year rate of cumulative risk of CNS disease was 1.6% (95% CI: 0.0-4.7) in 83 patients with and 3.4% (95% CI: 2.4-4.4) in 1252 patients without ECFI (P = .682; Figure 3). A multivariable analysis using time to CNS event as an end point (supplemental Table 9) showed a (nonsignificantly) increased HR for ECFI of 1.6 for all patients with ECFI and 1.7 for patients treated without rituximab, but there was no difference for patients treated with rituximab (HR = 1.0). If bulky disease was added to this model, the HR for ECFI was 1.7 (P = .130) for patients treated without and 1.0 (P = .977) for patients treated with rituximab. A multivariable analysis adjusting rituximab for IPI risk factors confirmed that the addition of rituximab reduced the relative risk for CNS disease in ECFI patients by 60% (HR = 0.4 [0.09; 2.1]); however, because of the small number of patients, this risk reduction did not reach significance (P = .302).
Time to CNS disease in patients with DLBCL and craniofacial involvement. There was a statistically nonsignificant trend toward increased CNS disease in the group with craniofacial involvement (n = 288) vs the group without craniofacial involvement (n = 3838) in the entire population (A; P = .122). There was a significant difference between 205 patients with and 2586 patients without craniofacial involvement in the patients treated without rituximab (B; P = .038). This difference disappeared and the 83 patients with craniofacial involvement developed less CNS disease than 1252 patients without when rituximab was given (C; P = .682). Gray dashed curves represent patients with and black curves represent patients without extralymphatic craniofacial involvement.
Time to CNS disease in patients with DLBCL and craniofacial involvement. There was a statistically nonsignificant trend toward increased CNS disease in the group with craniofacial involvement (n = 288) vs the group without craniofacial involvement (n = 3838) in the entire population (A; P = .122). There was a significant difference between 205 patients with and 2586 patients without craniofacial involvement in the patients treated without rituximab (B; P = .038). This difference disappeared and the 83 patients with craniofacial involvement developed less CNS disease than 1252 patients without when rituximab was given (C; P = .682). Gray dashed curves represent patients with and black curves represent patients without extralymphatic craniofacial involvement.
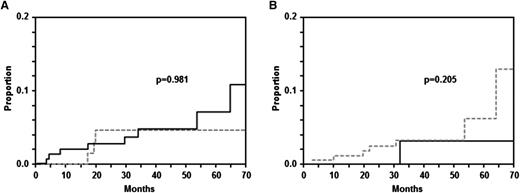
Information on intrathecal prophylaxis with MTX is available for 279/288 patients with ECFI. Of these 279 patients, 88 (31.5%) received intrathecal prophylaxis with MTX. The 2-year-rate of cumulative risk of CNS disease was 4.2% (95% CI: 0.0-8.9) in these patients compared with 2.3% (95% CI: 0.1-4.5) in 191 patients without prophylaxis (P = .981; Figure 4A). A multivariable analysis adjusting for IPI risk factors also showed that there was no difference between patients receiving or not MTX prophylaxis (HR = 0.9 [95% CI 0.2-3.4]; P = .828). Similarly, there was no difference in the incidence of CNS disease between patients who received radiotherapy to ECFI sites and those who did not. One patient who presented with synchronous peripheral and CNS disease was excluded from this analysis. Of 201 patients 144 received radiotherapy and 57 did not. The 2-year CNS rate was 0% in the latter and 2.2% (0; 4.7; P = .205) in patients who received radiotherapy (Figure 4B).
Time to CNS disease in patients with craniofacial involvement. (A) Patients receiving intrathecal prophylaxis with MTX prophylaxis or not. (B) Patients receiving radiotherapy or not. (A) There was no difference with respect to the rate of CNS disease between 88 patients with craniofacial involvement who received MTX prophylaxis (gray curve) and 191 patients who did not (black curve; P = .981). (B) Similarly, there was no difference in CNS incidence between 144 who received radiotherapy (gray curve) and 57 who did not (black curve; P = .205).
Time to CNS disease in patients with craniofacial involvement. (A) Patients receiving intrathecal prophylaxis with MTX prophylaxis or not. (B) Patients receiving radiotherapy or not. (A) There was no difference with respect to the rate of CNS disease between 88 patients with craniofacial involvement who received MTX prophylaxis (gray curve) and 191 patients who did not (black curve; P = .981). (B) Similarly, there was no difference in CNS incidence between 144 who received radiotherapy (gray curve) and 57 who did not (black curve; P = .205).
Discussion
To the best of our knowledge, this is the first and largest study of craniofacial involvement of DLBCL restricted to prospective trials in the rituximab era. Cases with involvement of the tonsils and Waldeyer ring were excluded from this analysis for 2 reasons: (1) because of the lymphatic tissue of origin, these cases should not behave biologically different from other (nodal) lymphatic structures, even though peculiarities mostly from their anatomic site have been reported,24,-26 and (2) because neither prophylaxis with intrathecal MTX nor radiotherapy to these sites (except for cases of bulky [≥7.5 cm] involvement of these lymphatic structures) were recommended, we did not include them in this study, the major objective of which was to analyze the role of prophylactic intrathecal MTX and radiotherapy to ECFI sites. A sensitivity analysis including the 731 patients with involvement of ECFI (290 patients) and the Waldeyer ring without ECFI (341 patients) yielded the same outcome results that we had obtained in patients with involvement of the other ECFI sites (data not shown).
Although grouping all ECFI sites that are quite heterogeneous into a single category improves statistical power, it risks missing identifying individual sites that may be associated with a higher risk of CNS relapse. However, because there were only 130 patients with a CNS event and only 14 CNS events occurred in the group of patients with ECFI, an analysis of subgroups of patients with ECFI is problematic. Nevertheless, when performing such an exploratory analysis according to the subgroups listed in supplemental Table 2, only the subgroup of patients with involvement of the paranasal sinuses had a higher risk for a CNS event (2-year rate: 6.4% in patients with paranasal sinuses vs 3.0% in patients without paranasal sinuses; P < .001). Only patients who had not received rituximab (2-year rate: 7.3% vs 2.9%, P < .001) contributed to this higher risk, in contrast to patients treated with rituximab, of whom only 4.3% with involvement of the paranasal sinuses had a CNS event compared with 3.2% without involvement of the paranasal sinuses (P = .303), demonstrating that the addition of rituximab abolishes the increased risk of CNS disease also in patients with involvement of the paranasal sinuses.
Although being the largest study addressing the role of radiotherapy and intrathecal prophylaxis in ECFI, our study has several limitations: (1) it is a retrospective study with all its caveats, selection bias being one of the most important; (2), despite the large number of total patients included this study, the number of patients with ECFI is still small, limiting the number of sensible subgroup analyses; and (3) the role of radiotherapy to ECFI sites and the issue of intrathecal prophylaxis were not addressed in a randomized fashion, but were determined by the policies of the institutions participating in the DSHNHL studies. We tried to avoid selection biases by including all ECFI sites and all previously published trials of the DSHNHL covering and representing all subgroups of DLBCL patients between 18 and 80 years of age; moreover, the patients included in this analysis are representative of all DLBCL patients treated in DSHNHL trials to date (data not shown). Finally, numerous multivariable analyses were performed to define the independent contribution of radiotherapy and intrathecal prophylaxis in ECFI patients.
One major finding of this study is that the prognosis of patients with ECFI is not different from other DLBCL patients in the rituximab era. Because ECFI was an independent favorable prognosticator (HR = 0.7; P = .010) for EFS in patients treated without rituximab (Table 2), the better EFS and OS of ECFI in patients treated without rituximab (Figure 1) was mostly because ECFI was associated with a favorable risk profile. When treated with rituximab, the outcome of patients with ECFI was not different from DLBCL patients without. This is not only due to the smaller number of patients treated with rituximab; rather, that rituximab quenches differences between prognostic subgroups by improving the outcome of poor-prognosis subgroups more than that of patients with low IPI appears to be responsible for this observation (Table 2).27
Our results do not support the generalizing conclusion of a Spanish study, that rituximab works better in lymphatic than extralymphatic disease28 ; rather, the results of this study and our recently published study of skeletal involvement29 suggest that differences in rituximab efficacy between lymphatic and extralymphatic sites may exist for some (eg, skeletal involvement), but not for other (eg, craniofacial) extralymphatic sites of involvement.
A second important finding of this study is the observation that radiotherapy to ECFI sites does not appear to improve the outcome of these patients (Figure 2), irrespective of whether the patients received rituximab or not. Although several groups have recommended combined chemoradiotherapy for lymphomas arising in the paranasal sinuses9,10,14,30,31 in the pre-rituximab era, this recommendation has never been scrutinized in prospective trials of patients treated with rituximab. Our results show that radiotherapy to sites of craniofacial involvement did not improve the outcome of these patients (Figure 2) or of patients treated with or without rituximab; this conclusion is supported by a multivariable analysis adjusting for the IPI risk factors (supplemental Table 6) that showed that radiotherapy did not change the hazard for all ECFI patients (HR = 1.0) or for patients in CR/CRu after immunochemotherapy (HR = 1.0). Patients in PR after immunochemotherapy also had an HR of 1.0; however, because 8/9 patients had received radiotherapy, the model for PR patients is less stable and we cannot exclude that patients in PR might benefit from additive radiotherapy to ECFI sites. In summary, the use of radiotherapy to ECFI sites, which can have negative effects on quality of life (xerostomia),32,33 is not supported by our data for patients in CR/CRu after immunochemotherapy. An analysis of the relapse pattern (supplemental Table 7) can serve as an explanation for this observation: 37/38 patients with ECFI (28/29 patients treated without rituximab and all 9/9 patients treated with rituximab) who relapsed did so at sites other than the original ECFI, irrespective of whether they had received radiotherapy to sites of primary ECFI. It is conceivable that local radiotherapy to ECFI sites would have hardly prevented these distant relapses. Interestingly, rituximab changed the relapse pattern considerably: although relapses developed more often in patients with ECFI with localized disease (66% vs 31%) in patients without rituximab, this ratio was reversed in patients with rituximab (22% vs 78%), indicating that rituximab reduced subclinical dissemination (in localized stages) very efficiently.
The third important finding of this study refers to CNS disease in ECFI. Because of their anatomical neighborhood, lymphomas of the paranasal sinuses carry a potential risk of spreading to the leptomeninges. This was confirmed in this study, yet only for patients without rituximab in which ECFI carried a significantly greater risk of CNS disease compared with patients without ECFI (P = .038). However, rituximab reduced the incidence of CNS events in both patients with and without ECFI, thus eliminating the differences in the CNS disease rate between patients with and without ECFI. This also holds true for paranasal sinuses when treated with rituximab, whereas frequency of CNS disease was increased in these patients when treated without rituximab. Of note, although the 2-year CNS rate was 2.2% in 144 patients who received ECFI irradiation, it was 0% in 57 ECFI patients who did not (Figure 4).
Because of the assumed increased risk of CNS disease in patients with ECFI, prophylactic intrathecal treatment with MTX has been recommended by several authors.9,10,30 However, our analysis shows that MTX prophylaxis did not reduce CNS disease in 88 patients with ECFI compared with 191 patients with ECFI who did not undergo intrathecal prophylaxis. These findings in patients with ECFI are similar to the observations made in the study of CNS disease in elderly DLBCL patients treated in the RICOVER-60 trial:34 intrathecal prophylaxis with MTX reduced the risk of patients treated without, but not in patients treated with, rituximab in the RICOVER-60 trial.
In summary, when treated with rituximab, the prognosis of patients with ECFI is not different from patients with other presentations of DLBCL. Similarly, the increased risk for CNS disease associated with ECFI, which was confirmed for the pre-rituximab era, has disappeared after the addition of rituximab; this is also the case for the subgroup of patients with involvement of the paranasal sinuses. The results of our study do not support intrathecal MTX or additive radiotherapy to ECFI sites as a prophylaxis to reduce the risk of CNS disease in patients with ECFI in CR/CRu outside prospective clinical studies in the rituximab era. However, because of the retrospective nature of this analysis, our results should be confirmed in a prospective trial. Whether positron emission tomography scans can identify those patients with ECFI who need or can be spared from radiotherapy might be a difficult question to address, because the predictive value of residual positivity involving osseous structures (including paranasal sinuses) is low because of false-positive cases caused by bone remodeling and inflammation,35 despite positron emission tomography scans having a high sensitivity for the detection of extralymphatic involvement by DLBCL,36 including the head and neck region.37
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
This work was supported by a grant from Deutsche Krebshilfe e.V. and Amgen, Spectrum, and Roche (M.P.).
Authorship
Contribution: N.M., S.Z., and M.P. designed the study; N.M., S.Z., M.P., M.Z., J.F., B.K., and C.R. analyzed the results; B.G., N.S., C.Z., A.V., M.H., M.W.-H., and R.M. provided clinical data; and all authors wrote the manuscript and approved the final version.
Conflict-of-interest disclosure: M.P. is a consultant or maintains an advisory role to Boehringer Ingelheim, Celgene, Gilead, Pfizer, and Onyx Roche. The remaining authors declare no competing financial interests.
Correspondence: Michael Pfreundschuh, Internal Medicine I, Saarland University Medical School, D-66421 Homburg (Saar), Germany; e-mail: michael.pfreundschuh@uks.eu.
References
Author notes
S.Z. and M.P. contributed equally to this study.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal