Key Points
Nilotinib induced deeper molecular responses than continued imatinib in patients with minimal residual disease on long-term imatinib.
These deeper responses may enable more patients to benefit from treatment-free remission trials.
Patients in complete cytogenetic response (CCyR) with detectable BCR-ABL1 after ≥2 years on imatinib were randomized to nilotinib (400 mg twice daily, n = 104) or continued imatinib (n = 103) in the Evaluating Nilotinib Efficacy and Safety in clinical Trials–Complete Molecular Response (ENESTcmr) trial. By 1 and 2 years, confirmed undetectable BCR-ABL1 was achieved by 12.5% vs 5.8% (P = .108) and 22.1% vs 8.7% of patients in the nilotinib and imatinib arms, respectively (P = .0087). Among patients without molecular response 4.5 (BCR-ABL1IS ≤0.0032%; MR4.5) and those without major molecular response at study start, MR4.5 by 2 years was achieved by 42.9% vs 20.8% and 29.2% vs 3.6% of patients in the nilotinib and imatinib arms, respectively. No patient in the nilotinib arm lost CCyR, vs 3 in the imatinib arm. Adverse events were more common in the nilotinib arm, as expected with the introduction of a new drug vs remaining on a well-tolerated drug. The safety profile of nilotinib was consistent with other reported studies. In summary, switching to nilotinib enabled more patients with chronic myeloid leukemia in chronic phase (CML-CP) to sustain lower levels of disease burden vs remaining on imatinib. This trial was registered at www.clinicaltrials.gov as #NCT00760877.
Introduction
Imatinib changed the paradigm for the treatment of chronic myeloid leukemia (CML) by establishing BCR-ABL1–targeted therapy as the standard of care for this disease.1,-3 However, even after long-term therapy (>5 years) with frontline imatinib, the majority of patients with CML treated with imatinib do not achieve stable undetectable BCR-ABL1 (with real-time quantitative polymerase chain reaction [RQ-PCR] with a sensitivity of ≥4.5 logs).4,5 Sustained deep molecular response (≥4.5-log reduction in BCR-ABL1 from a standardized baseline) is an eligibility requirement for most treatment-free remission (TFR) studies. In the Evaluating Nilotinib Efficacy and Safety in clinical Trials–Newly Diagnosed Patients (ENESTnd) study, significantly more patients treated with nilotinib achieved major molecular response (MMR) and a molecular response 4.5-log reduction from a standardized baseline (MR4.5; BCR-ABL1 on the International Scale [IS] ≤0.0032%) compared with imatinib.6,7 These deeper molecular responses achieved with nilotinib may enable more patients with CML to participate in ongoing TFR studies.
ENEST–Complete Molecular Response (ENESTcmr) is an open-label, randomized, prospective, multicenter, phase 3 trial of continued imatinib vs a switch to nilotinib in patients with detectable BCR-ABL1 after at least 2 years on imatinib. Herein, we report results of ENESTcmr with up to 2 years of follow-up of all patients.
Patients, materials, and methods
Study design and treatments
Patients were randomized 1:1 to receive either nilotinib (400 mg twice daily) or imatinib (400 or 600 mg once daily; same dose as was received before randomization). Eligible adults (aged ≥18 years) had Philadelphia chromosome–positive (Ph+) CML in chronic phase (CML-CP) and were in complete cytogenetic response (CCyR; documented by standard cytogenetics or peripheral blood BCR-ABL1IS <1%) but had persistent minimal residual disease (MRD) after ≥2 years on imatinib (400 mg or 600 mg daily). Persistent MRD was defined by 2 positive RQ-PCR tests in local laboratories at least 8 weeks apart, with at least 1 performed within 3 months of randomization.
Only patients who tolerated imatinib well were enrolled in the study. Patients were excluded if they had a change in imatinib dose within 6 months of study entry or major toxicity on imatinib within 3 months of study entry.
PCR methodology
Samples for molecular end points were collected every 3 months. RNA was stabilized within 24 hours of sample collection into TRIzol stabilization solution. The samples were stored frozen and batch shipped to a central laboratory in Adelaide, Australia, where all samples were evaluated by RQ-PCR.8 The process was implemented to achieve 2 goals: to ensure high sample sensitivity (early stabilization of RNA) and to eliminate interlaboratory variability by processing samples in a central laboratory. BCR-ABL1 kinase domain mutation analysis was performed using direct sequencing for a significant BCR-ABL1 rise.9 If mutations were detected, prior samples were tested to determine when the mutation was first detectable.
End points
The primary end point of this study was the rate of confirmed best cumulative undetectable BCR-ABL1 by 12 months. Confirmed undetectable BCR-ABL1 was defined as undetectable BCR-ABL1 by RQ-PCR with a sample sensitivity of at least 4.5 logs below the standardized baseline, as expressed on the IS. RQ-PCR negativity was confirmed in the next RQ-PCR sample with sensitivity of at least 4 logs. Results were expressed as the proportion of patients who achieved confirmed best cumulative undetectable BCR-ABL1 by 12 months over the intention-to-treat (ITT) population. Secondary end points included determination of event-free survival (EFS) and safety profile in the 2 study arms. EFS was defined as the time from the date of randomization to the date of the first occurrence of any of the following: death from any cause during treatment, progression to accelerated phase/blast crisis, or confirmed loss of response (complete hematologic response, CCyR, or MMR) in 2 consecutive assessments.
Post hoc analyses included determination of molecular response (BCR-ABL1IS ≤0.0032% [MR4.5], undetectable BCR-ABL1) over time in patients without these levels of response at study start and evaluation of cumulative molecular responses according to patients’ responses at study start. Cumulative responses were reported as proportions (patients with the response of interest by a particular time point/all patients without the response of interest at baseline). Cumulative incidence graphs are presented according to the Kaplan-Meier method.
Statistical analysis
Unless otherwise noted, all statistical tests were conducted against a 2-sided alternative hypothesis with a significance level of 0.05. The ITT population consisted of all randomized patients. The safety population consisted of all patients who received at least 1 dose of study drug and had at least 1 safety assessment. The primary efficacy analysis was performed using a Cochran-Mantel-Haenszel test, accounting for the randomization strata on the ITT population. The Kaplan-Meier method was used to summarize time-to-event end points. For all secondary, exploratory, and post hoc end points reported here, P values were provided for descriptive purposes and were not adjusted for multiple comparisons.
Ethics and study management
This study was conducted in accordance with the Declaration of Helsinki. The protocol was approved by review boards at all participating institutions. Written informed consent was obtained from each patient. This trial was registered at www.clinicaltrials.gov as #NCT00760877.
Results
Patients and treatments
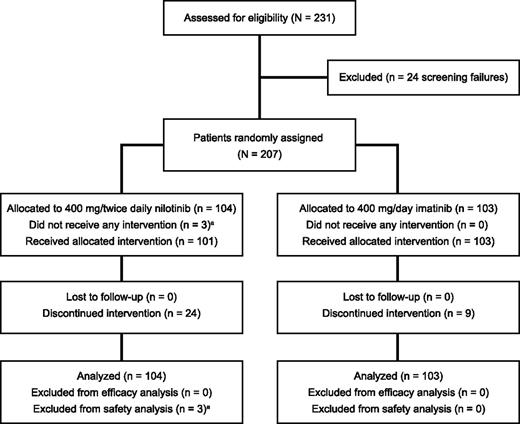
From June 12, 2009, to June 30, 2010, a total of 207 patients with CML-CP with persistent MRD after at least 2 years of imatinib therapy were randomized (Figure 1). For this analysis, data were cut for each patient at the earlier of the discontinuation date or the date of their month 24 visit. Characteristics at study start were comparable between the nilotinib and imatinib arms (Table 1). Approximately 80% of patients had received more than 3 years of prior imatinib. Two patients on each arm were included in the study who had detectable BCR-ABL1 at study start in the local laboratory but achieved undetectable BCR-ABL1 in the central laboratory assessment.
Patient characteristics (intention-to-treat population)
| . | Nilotinib 400 mg twice daily (n = 104) . | Imatinib 400-600 mg once daily (n = 103) . |
|---|---|---|
| Median age (range), y | 46 (23-82) | 52 (19-76) |
| Sex, n (%) | ||
| Male | 71 (68.3) | 65 (63.1) |
| Female | 33 (31.7) | 38 (36.9) |
| Race or ethnic group, n (%) | ||
| White | 88 (84.6) | 80 (77.7) |
| Black | 5 (4.8) | 3 (2.9) |
| Asian | 4 (3.8) | 0 |
| Hispanic | 3 (2.9%) | 8 (7.8) |
| Other | 4 (3.8%) | 12 (11.7) |
| Prior duration of imatinib, n (%) | ||
| ≤36 mo | 18 (17.3) | 21 (20.4) |
| >36 mo | 86 (82.7) | 82 (79.6) |
| Prior interferon use, n (%) | ||
| None | 59 (56.7) | 57 (55.3) |
| ≤12 mo | 21 (20.2) | 22 (21.3) |
| >12 mo | 24 (23.1) | 24 (23.3) |
| Responses at study start, n (%) | ||
| Missing | 1 (1.0) | 1 (1.0) |
| CCyR but no MMR (BCR-ABL1IS ≤1% and >0.1) | 24 (23.1) | 28 (27.2) |
| MMR but no MR4 (BCR-ABL1IS ≤0.1% and >0.01) | 50 (48.1) | 50 (48.5) |
| MR4 but no MR4.5 (BCR-ABL1IS ≤0.01% and >0.0032%) | 24 (23.1) | 18 (17.5) |
| MR4.5 but not undetectable BCR-ABL1 (BCR-ABL1IS ≤0.0032% and >0.00001%) | 3 (2.9) | 4 (3.9) |
| Undetectable BCR-ABL1* | 2 (1.9) | 2 (1.9) |
| . | Nilotinib 400 mg twice daily (n = 104) . | Imatinib 400-600 mg once daily (n = 103) . |
|---|---|---|
| Median age (range), y | 46 (23-82) | 52 (19-76) |
| Sex, n (%) | ||
| Male | 71 (68.3) | 65 (63.1) |
| Female | 33 (31.7) | 38 (36.9) |
| Race or ethnic group, n (%) | ||
| White | 88 (84.6) | 80 (77.7) |
| Black | 5 (4.8) | 3 (2.9) |
| Asian | 4 (3.8) | 0 |
| Hispanic | 3 (2.9%) | 8 (7.8) |
| Other | 4 (3.8%) | 12 (11.7) |
| Prior duration of imatinib, n (%) | ||
| ≤36 mo | 18 (17.3) | 21 (20.4) |
| >36 mo | 86 (82.7) | 82 (79.6) |
| Prior interferon use, n (%) | ||
| None | 59 (56.7) | 57 (55.3) |
| ≤12 mo | 21 (20.2) | 22 (21.3) |
| >12 mo | 24 (23.1) | 24 (23.3) |
| Responses at study start, n (%) | ||
| Missing | 1 (1.0) | 1 (1.0) |
| CCyR but no MMR (BCR-ABL1IS ≤1% and >0.1) | 24 (23.1) | 28 (27.2) |
| MMR but no MR4 (BCR-ABL1IS ≤0.1% and >0.01) | 50 (48.1) | 50 (48.5) |
| MR4 but no MR4.5 (BCR-ABL1IS ≤0.01% and >0.0032%) | 24 (23.1) | 18 (17.5) |
| MR4.5 but not undetectable BCR-ABL1 (BCR-ABL1IS ≤0.0032% and >0.00001%) | 3 (2.9) | 4 (3.9) |
| Undetectable BCR-ABL1* | 2 (1.9) | 2 (1.9) |
MR4 molecular response 4-log reduction from a standardized baseline.
With a sample sensitivity of ≥4.5 logs.
Overall, 1692 samples were analyzed by RQ-PCR. In both arms, median sensitivity for all samples was 4.7 logs. Median time to RNA stabilization was 22.4 hours in the imatinib arm and 23.6 hours in the nilotinib arm.
Median relative dose intensities were 0.98 (median dose intensity, 782.6 mg/day), 1.00 (median dose intensity, 400 mg/day), and 1.00 (median dose intensity, 600 mg/day) for patients who received nilotinib 400 mg twice daily, imatinib 400 mg once daily, and imatinib 600 mg once daily, respectively. At 2 years, 80 (76.9%) and 94 (91.3%) patients in the nilotinib and imatinib arms, respectively, were still receiving study treatment (Table 2; P = .0025). The most common reason for discontinuation was adverse events (AEs) in 12 patients (11.5%) in the nilotinib arm and 3 (2.9%) in the imatinib arm.
Patient disposition
| Patients, n (%) . | Nilotinib 400 mg twice daily (n = 104) . | Imatinib 400-600 mg once daily (n = 103) . |
|---|---|---|
| Still on treatment | 80 (76.9) | 94 (91.3) |
| Discontinued | 24 (23.1) | 9 (8.7) |
| AE(s) | 12 (11.5) | 3 (2.9) |
| Withdrawal of consent | 6 (5.8) | 3 (2.9) |
| Death | 1 (1.0)* | 0 |
| Other† | 5 (4.8) | 3 (2.9) |
| Patients, n (%) . | Nilotinib 400 mg twice daily (n = 104) . | Imatinib 400-600 mg once daily (n = 103) . |
|---|---|---|
| Still on treatment | 80 (76.9) | 94 (91.3) |
| Discontinued | 24 (23.1) | 9 (8.7) |
| AE(s) | 12 (11.5) | 3 (2.9) |
| Withdrawal of consent | 6 (5.8) | 3 (2.9) |
| Death | 1 (1.0)* | 0 |
| Other† | 5 (4.8) | 3 (2.9) |
A 59-year-old male patient with a medical history of diabetes, hyperlipidemia, hypertension, and obesity who died due to suspected acute myocardial infarction.
Includes nonadherence with protocol or treatment, unacceptable toxicity, protocol violation, and transient loss of MMR (n = 1 each on nilotinib), pregnancy (imatinib), and unspecified (n = 1 nilotinib, n = 2 imatinib).
Efficacy
The primary efficacy end point of cumulative rate of confirmed undetectable BCR-ABL1 by 1 year was 12.5% vs 5.8% for the nilotinib and imatinib arms, respectively (P = .1083). By 2 years, the cumulative rate of confirmed undetectable BCR-ABL1 was higher in the nilotinib arm (22.1% vs 8.7%; odds ratio, 2.565; 95% confidence interval [CI], 1.107 to 5.947; P = .0087). The increase in the proportion of patients with undetectable BCR-ABL1 from months 12 to 24 was higher in the nilotinib arm (9.6 percentage points) vs the imatinib arm (2.9 percentage points).
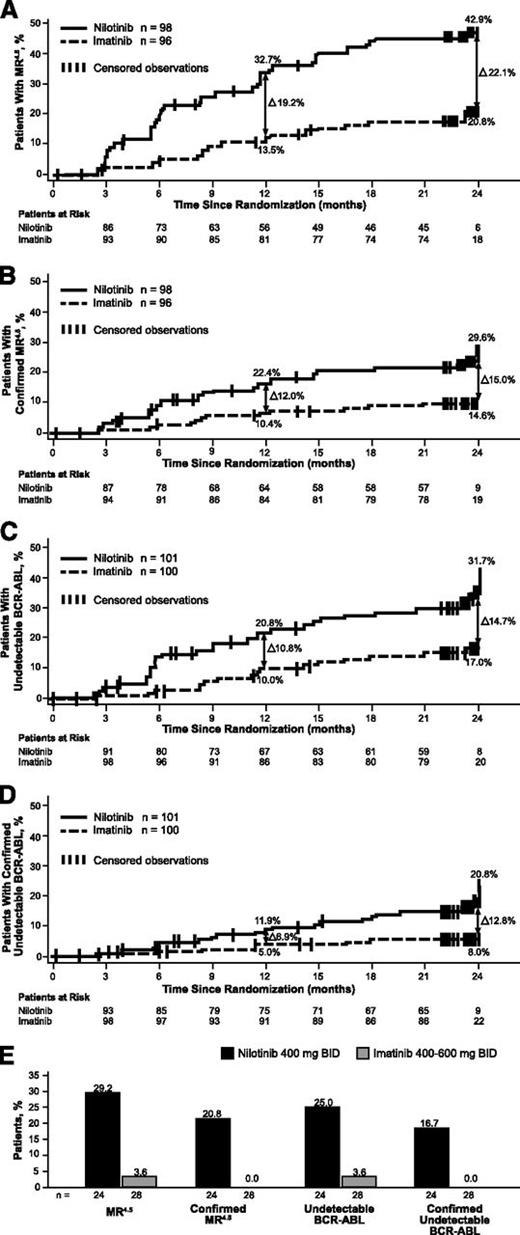
The cumulative incidences of molecular responses by 2 years were evaluated in post hoc analyses according to patients’ responses on imatinib at study start. Rates of deep molecular response were higher in the nilotinib arm regardless of response at study start. MR4.5 was achieved by 42.9% (42/98) vs 20.8% (20/96) of patients not in MR4.5 at study start in the nilotinib and imatinib arms, respectively (Figure 2A). Confirmed MR4.5 was achieved in 29.6% and 14.6% of patients not in MR4.5 at study start in the nilotinib and imatinib arms, respectively (Figure 2B). Undetectable BCR-ABL1 was achieved by 31.7% (32/101) vs 17.0% (17/100) of patients with documented detectable BCR-ABL1 at study start in the nilotinib and imatinib arms (Figure 2C). Confirmed undetectable BCR-ABL1 was achieved by 20.8% and 8.0% with documented detectable BCR-ABL1 at study start, respectively (Figure 2D).
Molecular responses. (A) Cumulative incidence of MR4.5 in patients without MR4.5 at study start. (B) Cumulative incidence of confirmed MR4.5 among patients without MR4.5 at study start. (C) Cumulative incidence of undetectable BCR-ABL1 in patients with detectable BCR-ABL1 at study start. (D) Cumulative incidence of confirmed undetectable BCR-ABL1 among patients with detectable BCR-ABL1 at study start. (E) Molecular responses in patients without MMR at study start.
Molecular responses. (A) Cumulative incidence of MR4.5 in patients without MR4.5 at study start. (B) Cumulative incidence of confirmed MR4.5 among patients without MR4.5 at study start. (C) Cumulative incidence of undetectable BCR-ABL1 in patients with detectable BCR-ABL1 at study start. (D) Cumulative incidence of confirmed undetectable BCR-ABL1 among patients with detectable BCR-ABL1 at study start. (E) Molecular responses in patients without MMR at study start.
Among patients who lacked MMR at study start, 83.3% (20/24) and 53.6% (15/28) achieved MMR on nilotinib and imatinib, respectively. Patients without MMR were more likely to achieve MR4.5 (29.2% vs 3.6%) and undetectable BCR-ABL1 (25.0% vs 3.6%) in the nilotinib than the imatinib arm (Figure 2E). Additionally, of the patients who were not in MMR at study start, no patients (0/28) in the imatinib arm achieved confirmed MR4.5 or confirmed undetectable BCR-ABL1 by 2 years, vs 20.8% (5/24) and 16.7% (4/24), respectively, in the nilotinib arm.
In patients with MMR but without MR4.5 at study start, a higher proportion achieved MR4.5 and undetectable BCR-ABL1 with nilotinib compared with imatinib; 47.3% (35/74) achieved MR4.5 by 24 months on nilotinib (vs 27.9% [19/68] on imatinib), and 33.8% (25/74) achieved undetectable BCR-ABL1 by 2 years on nilotinib (vs 22.1% [15/68] on imatinib). Similarly, among patients with MMR but without undetectable BCR-ABL1 at study start, more patients on nilotinib (vs imatinib) achieved undetectable BCR-ABL1 (33.8% [26/77] vs 22.2% [16/72]).
There was no clear association between achievement of MR4.5 and prior dose of imatinib. In the per-protocol population (n = 101 for nilotinib, n = 59 for prior imatinib 400 mg once daily, n = 44 for prior imatinib 600 mg once daily), 39.6%, 25.4%, and 18.2% of patients in the nilotinib, prior imatinib 400 mg once daily, and prior imatinib 600 mg once daily arms achieved MR4.5 by 1 year, respectively.
By 2 years, no patient in either arm had progressed to accelerated phase/blast crisis. Stable MR4.5 was reported in 12 (11.5%) patients in the nilotinib arm and 6 (5.8%) in the imatinib arm. A total of 3 patients in the nilotinib arm (n = 2 confirmed loss of MMR; n = 1 death) and 7 in the imatinib arm (n = 4 confirmed loss of MMR; n = 3 confirmed loss of CCyR) had an event within the first 2 years on treatment. The estimated rates of EFS at 24 months on nilotinib and imatinib were 96.6% (95% CI, 89.8% to 98.9%) vs 92.8% (95% CI, 85.5% to 96.5%), respectively. Median EFS was not yet reached for either treatment arm.
Six patients had BCR-ABL1 mutations detected (Table 3), 1 in the nilotinib arm and 5 in the imatinib arm. Only 1 of these patients was in MMR at study entry. In 2 patients, the mutation was known to be present at study start (1 randomized to nilotinib and 1 to imatinib), whereas the other 4 were tested due to a significant BCR-ABL1 rise (all randomized to imatinib), with 3 showing positivity for mutations in samples collected at study start. Thus, 5 of 6 patients with mutations had the mutation at study start. All 3 patients who lost CCyR in the imatinib arm had a mutation. None of the 5 patients with mutations randomized to imatinib achieved the primary end point, whereas the patient with the mutation at study start in the nilotinib arm did achieve this response.
Mutations detected at study start or on study treatment
| Arm/mutation . | Present at study start . | Response on study drug . | Treatment after mutation detection . |
|---|---|---|---|
| Nilotinib | |||
| E450K | Yes | Stable undetectable BCR-ABL1 | Remained on nilotinib |
| Imatinib | |||
| E255K | Yes | Fluctuating BCR-ABL1 levels | Crossed over to nilotinib |
| L248V | Yes | No MMR | Remained on imatinib |
| G250E/F359V | Yes | Loss of CCyR | Crossed over to nilotinib |
| G250E | Yes | Loss of CCyR | Crossed over to nilotinib |
| E453K | No | Loss of CCyR | Crossed over to nilotinib |
| Arm/mutation . | Present at study start . | Response on study drug . | Treatment after mutation detection . |
|---|---|---|---|
| Nilotinib | |||
| E450K | Yes | Stable undetectable BCR-ABL1 | Remained on nilotinib |
| Imatinib | |||
| E255K | Yes | Fluctuating BCR-ABL1 levels | Crossed over to nilotinib |
| L248V | Yes | No MMR | Remained on imatinib |
| G250E/F359V | Yes | Loss of CCyR | Crossed over to nilotinib |
| G250E | Yes | Loss of CCyR | Crossed over to nilotinib |
| E453K | No | Loss of CCyR | Crossed over to nilotinib |
AEs
Any-grade and grade 3/4 serious AEs of any causality occurred in a similar proportion of patients in the nilotinib and imatinib arms (Table 4). The most common drug-related AEs in the nilotinib arm were headache (33.7%), rash (27.7%), and pruritus (23.8%; Tables 5 and 6). In the imatinib arm, 63 patients (61.2%) had drug-related AEs; 6 (5.8%) with grade 3/4 AEs. The most common were muscle spasms (12.6%), nausea (11.7%), and diarrhea (10.7%).
Summary of AEs
| . | Nilotinib (n = 101) . | Imatinib (n = 103) . | ||
|---|---|---|---|---|
| . | All grades, n (%) . | Grade 3/4, n (%) . | All grades n (%) . | Grade 3/4, n (%) . |
| AEs | 100 (99.0) | 49 (48.5) | 93 (90.3) | 23 (22.3) |
| Serious AEs | 18 (17.8) | 14 (13.9) | 13 (12.6) | 11 (10.7) |
| AEs leading to discontinuation | 20 (19.8) | 10 (9.9) | 6 (5.8) | 4 (3.9) |
| AEs requiring dose interruption and/or change | 59 (58.4) | 32 (31.7) | 17 (16.5) | 6 (5.8) |
| . | Nilotinib (n = 101) . | Imatinib (n = 103) . | ||
|---|---|---|---|---|
| . | All grades, n (%) . | Grade 3/4, n (%) . | All grades n (%) . | Grade 3/4, n (%) . |
| AEs | 100 (99.0) | 49 (48.5) | 93 (90.3) | 23 (22.3) |
| Serious AEs | 18 (17.8) | 14 (13.9) | 13 (12.6) | 11 (10.7) |
| AEs leading to discontinuation | 20 (19.8) | 10 (9.9) | 6 (5.8) | 4 (3.9) |
| AEs requiring dose interruption and/or change | 59 (58.4) | 32 (31.7) | 17 (16.5) | 6 (5.8) |
Most commonly reported (≥5%) drug-related nonhematologic AEs
| Nonhematologic . | Nilotinib 400 mg twice daily (n = 101) . | Imatinib 400-600 mg once daily (n = 103) . | ||
|---|---|---|---|---|
| All grades, n (%) . | Grade 3/4, n (%) . | All grades, n (%) . | Grade 3/4, n (%) . | |
| Headache | 34 (33.7) | 2 (2.0) | 2 (1.9) | 0 |
| Rash | 28 (27.7) | 1 (1.0) | 2 (1.9) | 0 |
| Pruritus | 24 (23.8) | 1 (1.0) | 0 | 0 |
| Abdominal pain* | 15 (14.9) | 0 | 6 (5.8) | 0 |
| Nausea | 15 (14.9) | 0 | 12 (11.7) | 0 |
| Fatigue | 14 (13.9) | 1 (1.0) | 3 (2.9) | 0 |
| Myalgia | 13 (12.9) | 1 (1.0) | 1 (1.0) | 0 |
| Muscle spasms | 11 (10.9) | 0 | 13 (12.6) | 0 |
| Constipation | 10 (9.9) | 1 (1.0) | 0 | 0 |
| Dry skin | 10 (9.9) | 0 | 0 | 0 |
| Pain in extremity | 9 (8.9) | 0 | 0 | 0 |
| Alopecia | 8 (7.9) | 0 | 0 | 0 |
| Arthralgia | 7 (6.9) | 0 | 2 (1.9) | 0 |
| Asthenia | 6 (5.9) | 0 | 2 (1.9) | 0 |
| Diarrhea | 6 (5.9) | 0 | 11 (10.7) | 0 |
| Folliculitis | 6 (5.9) | 0 | 0 | 0 |
| Insomnia | 6 (5.9) | 0 | 2 (1.9) | 0 |
| Decreased appetite | 5 (5.0) | 0 | 3 (2.9) | 0 |
| Hyperglycemia | 5 (5.0) | 0 | 4 (3.9) | 0 |
| Vomiting | 5 (5.0) | 1 (1.0) | 4 (3.9) | 0 |
| Nonhematologic . | Nilotinib 400 mg twice daily (n = 101) . | Imatinib 400-600 mg once daily (n = 103) . | ||
|---|---|---|---|---|
| All grades, n (%) . | Grade 3/4, n (%) . | All grades, n (%) . | Grade 3/4, n (%) . | |
| Headache | 34 (33.7) | 2 (2.0) | 2 (1.9) | 0 |
| Rash | 28 (27.7) | 1 (1.0) | 2 (1.9) | 0 |
| Pruritus | 24 (23.8) | 1 (1.0) | 0 | 0 |
| Abdominal pain* | 15 (14.9) | 0 | 6 (5.8) | 0 |
| Nausea | 15 (14.9) | 0 | 12 (11.7) | 0 |
| Fatigue | 14 (13.9) | 1 (1.0) | 3 (2.9) | 0 |
| Myalgia | 13 (12.9) | 1 (1.0) | 1 (1.0) | 0 |
| Muscle spasms | 11 (10.9) | 0 | 13 (12.6) | 0 |
| Constipation | 10 (9.9) | 1 (1.0) | 0 | 0 |
| Dry skin | 10 (9.9) | 0 | 0 | 0 |
| Pain in extremity | 9 (8.9) | 0 | 0 | 0 |
| Alopecia | 8 (7.9) | 0 | 0 | 0 |
| Arthralgia | 7 (6.9) | 0 | 2 (1.9) | 0 |
| Asthenia | 6 (5.9) | 0 | 2 (1.9) | 0 |
| Diarrhea | 6 (5.9) | 0 | 11 (10.7) | 0 |
| Folliculitis | 6 (5.9) | 0 | 0 | 0 |
| Insomnia | 6 (5.9) | 0 | 2 (1.9) | 0 |
| Decreased appetite | 5 (5.0) | 0 | 3 (2.9) | 0 |
| Hyperglycemia | 5 (5.0) | 0 | 4 (3.9) | 0 |
| Vomiting | 5 (5.0) | 1 (1.0) | 4 (3.9) | 0 |
Includes the preferred terms abdominal pain and abdominal pain, upper.
Most commonly reported (≥5%) newly occurring or worsening grade 3/4 hematologic and biochemical abnormalities
| . | Nilotinib 400 mg twice daily (n = 101) . | Imatinib 400-600 mg once daily (n = 103) . |
|---|---|---|
| Newly occurring or worsening grade 3/4 hematologic laboratory abnormalities, n (%) | ||
| Anemia* | 1 (1.0) | 1 (1.0) |
| Thrombocytopenia | 0 | 1 (1.0) |
| Neutropenia | 2 (2.0) | 4 (3.9) |
| Newly occurring or worsening grade 3/4 biochemical abnormalities, n (%) | ||
| Decreased phosphate | 12 (11.9) | 18 (17.5) |
| Elevated lipase | 10 (9.9) | 1 (1.0) |
| Elevated bilirubin (total)† | 5 (5.0) | 0 |
| Increase alanine aminotransferase | 4 (4.0) | 0 |
| Decreased potassium | 0 | 1 (1.0) |
| Decreased magnesium | 0 | 1 (1.0) |
| . | Nilotinib 400 mg twice daily (n = 101) . | Imatinib 400-600 mg once daily (n = 103) . |
|---|---|---|
| Newly occurring or worsening grade 3/4 hematologic laboratory abnormalities, n (%) | ||
| Anemia* | 1 (1.0) | 1 (1.0) |
| Thrombocytopenia | 0 | 1 (1.0) |
| Neutropenia | 2 (2.0) | 4 (3.9) |
| Newly occurring or worsening grade 3/4 biochemical abnormalities, n (%) | ||
| Decreased phosphate | 12 (11.9) | 18 (17.5) |
| Elevated lipase | 10 (9.9) | 1 (1.0) |
| Elevated bilirubin (total)† | 5 (5.0) | 0 |
| Increase alanine aminotransferase | 4 (4.0) | 0 |
| Decreased potassium | 0 | 1 (1.0) |
| Decreased magnesium | 0 | 1 (1.0) |
Five cases of anemia were reported in the nilotinib arm and 2 in the imatinib arm, but 4 in the nilotinib arm and 1 in the imatinib arm were determined to be a result of errors in unit conversion. None of these patients actually had hemoglobin values consistent with anemia.
Includes the preferred terms hyperbilirubinemia and increased blood bilirubin.
Drug-related AEs that led to study discontinuation were reported in 14 patients in the nilotinib arm (7 grade 1/2 [64.3%]; 6 grade 3; 1 grade 4). In the imatinib arm, 1 patient discontinued due to grade 4 progression of a prostate tumor, 1 patient discontinued due to progression of chronic lymphocytic leukemia, and 1 patient discontinued due to grade 3 gastrointestinal hemorrhage.
A 59-year-old male patient with a medical history of obesity, hypertension, and diabetes who was randomized to nilotinib died after 6 months on study due to suspected acute myocardial infarction. Ischemic heart disease was observed in 4 patients (3 in the nilotinib arm; 1 in the imatinib arm). Peripheral arterial disease (PAD) was observed in 3 patients (all in the nilotinib arm); all 3 had preexisting risk factors. No patient in either treatment arm had an increase >60 ms in QTcF over levels at study start or an absolute QTcF prolongation >480 ms.
Discussion
In patients with detectable disease and varying degrees of molecular responses on long-term imatinib, switching to nilotinib was more effective at inducing deeper molecular responses than remaining on imatinib. The cumulative incidences of MR4.5 (in patients without MR4.5 at study start) and undetectable BCR-ABL1 (in patients with detectable BCR-ABL1 at study start) were each higher in the nilotinib arm compared with the imatinib arm. Patients in the nilotinib arm were also more likely to have confirmed responses and stable MR4.5.
ENESTcmr used highly sensitive, reproducible molecular testing. Median sensitivity for all RQ-PCR samples was high at 4.7 logs and was consistent between arms. Median time to RNA stabilization was also consistent and was less than 24 hours in both arms, in keeping with current best practices. To ensure consistency and optimal results, all samples were analyzed in a central laboratory. This sensitivity and reproducibility is important when evaluating “undetectable” disease, and these results demonstrate that the differences between the nilotinib and imatinib arms were real and not artifacts of improper or differential sample handling.
The deeper responses observed with switch to nilotinib vs remaining on imatinib are consistent with those reported in the ENESTnd study,6,7 which showed continued superiority for nilotinib in inducing deep molecular responses over imatinib after 5 years of follow-up; these data were especially dramatic when evaluating deeper levels of response such as MR4.5. The ability of nilotinib to induce deep molecular responses was particularly pronounced in patients who lacked MMR at study start. Rates of MR4.5 and undetectable BCR-ABL1 were low in patients without MMR at study start who remained on imatinib, none of whom achieved confirmed responses at these molecular levels. Patients with good responses (at least MMR) at study start also benefitted from switching to nilotinib. A higher proportion of patients with MMR (but without MR4.5 or undetectable BCR-ABL1) achieved MR4.5 and undetectable BCR-ABL1, respectively, on nilotinib vs imatinib. Thus, a substantial proportion of patients both with and without MMR on long-term imatinib achieved deeper molecular responses by switching to nilotinib. Notably, at the time of data cutoff, the vast majority of patients in the imatinib arm had received imatinib for over 5 years: more than 3 years prior to study entry and 2 years on study. These data suggest that longer treatment with imatinib is unlikely to result in the deepest levels of molecular responses in patients who have achieved CCyR or MMR but not deeper molecular responses on long-term imatinib.
Although the trial failed to meet its primary end point, a twofold increase in the rate of confirmed undetectable BCR-ABL1 by 1 year was reported with nilotinib vs imatinib. The lack of statistical significance at this early 1-year time point may be related to the kinetics of molecular response with nilotinib in this patient population, which were largely unknown at the time of the design of this study. The rates of MR4.5 and undetectable BCR-ABL1 were higher in the nilotinib arm throughout the study. Between 12 and 24 months, the difference in cumulative rate of confirmed undetectable BCR-ABL grew between the nilotinib and imatinib arms and was higher in the nilotinib arm by 24 months. This trend was consistently observed in all end points evaluated in both unconfirmed and confirmed response analyses. This is consistent with the responses observed in ENESTnd: the difference in the cumulative rates of MR4.5 by 1 year for nilotinib 400 mg twice daily over imatinib 400 mg once daily was 6% and has continued to widen with longer follow-up (10% by 2 years, 13% by 3 years, 14% by 4 years, 21% by 5 years).6,7,10 Based on these findings, we now conclude that the primary assessment of ENESTcmr should have been performed not at 12 months but at a later time point, such as at 24 months.
In addition, several recent landmark analyses demonstrated that achievement of the deepest levels of molecular response (confirmed MR4.5 and undetectable BCR-ABL1) on tyrosine kinase inhibitor (TKI) therapy was associated with significantly improved long-term outcomes, including EFS, transformation-free survival, and failure-free survival.11,-13 In the German CML-IV study, for example, increasing levels of molecular response (BCR-ABL1 <0.0032%, 0.0032% to 0.01%, 0.01% to 0.1%, 0.1% to 1%, >1%) at 4 years was associated with increasingly higher rates of OS at 8 years, regardless of treatment approach. Furthermore, in the CML-IV study, there were 6 deaths and no progression events in patients with MR4.5, vs 42 deaths and 9 progressions in patients who achieved MMR.11 In a prospective analysis of French patients with CML treated with frontline imatinib, patients who achieved complete molecular response (defined as MR4.5 with undetectable BCR-ABL transcripts at 2 consecutive assessments >2 months apart) had significantly improved EFS and failure-free survival vs patients who achieved MMR.13 Together, these studies demonstrated that patients who achieve MR4.5 or other deeper molecular responses have significantly improved long-term outcomes compared with patients without these responses, including those who achieve MMR.
Patients experienced AEs early on nilotinib after long-term imatinib therapy in a pattern consistent with the observed safety profile of nilotinib in other studies of patients who switched to nilotinib after imatinib failure.14,,-17 The relative median dose intensity was almost 100% of planned dosing with nilotinib. Patients in ENESTcmr were a preselected population already tolerating imatinib therapy. Patients were required to have received treatment with imatinib for a minimum of 2 years, with no imatinib dose adjustment within 6 months and no major toxicity within 3 months of study entry. The incidence of AEs, including grade 3/4 AEs, was higher among patients who switched to nilotinib vs those who remained on imatinib, but the incidence of serious AEs was similar between the 2 arms. AEs most commonly included headache, rash, and pruritus with nilotinib and muscle spasms, nausea, and diarrhea with imatinib. The appearance of AEs in patients who switched to nilotinib was not surprising as it was associated with introduction of a new drug vs remaining on an already well-tolerated drug. Ischemic heart disease and PAD were infrequent, but more cases were reported in the nilotinib arm (3 cases of ischemic heart disease and 3 cases of PAD vs 1 and 0 in the imatinib arm). All 3 patients with PAD had preexisting cardiovascular disease risk factors. Nearly two-thirds of AEs that led to discontinuation for patients who switched from imatinib to nilotinib were grade 1/2. Three patients (2.9%) in the imatinib arm discontinued due to intolerance, showing that AEs can also occur in patients stabilized on long-term imatinib.
In patients with molecular responses less than MR4.5, the clinical benefits of switching to nilotinib to achieve deeper molecular responses should be carefully weighed against the potential for AEs, including cardiovascular AEs, on an individual basis. An AE of any grade that requires a switch in therapy may be considered a competing risk event for the achievement of response on that TKI,18 and management of AEs arising on TKI therapy is recommended according to current guidelines.19 Proper adherence to therapy is essential, as nonadherence can contribute to a reduced ability to achieve molecular responses, a loss of CCyR, and the occurrence of AEs.20,-22 For physicians involved in the treatment of CML, AEs that may occur after TKI switch must be viewed in light of the fact that patients who achieve deeper responses such as MR4.5 are likely to have better long-term outcomes.11,-13
The risk of events on imatinib, such as loss of response, must also be considered. Significantly fewer patients who remained on imatinib vs switching to nilotinib went on to achieve deeper molecular responses or stable deep molecular responses in ENESTcmr. Three patients lost CCyR by 24 months on imatinib, whereas no patients experienced a loss of CCyR after switch to nilotinib. After 7 years of follow-up of patients treated with frontline imatinib in IRIS, 456 patients had achieved CCyR; of these patients, 79 lost CCyR (17%).23 Consistent with this observation, a landmark analysis of patients treated with frontline imatinib in IRIS demonstrated that patients who achieved CCyR but not MMR by 18 months had a significantly higher risk of loss of CCyR by 7 years compared with patients who achieved CCyR and MMR (26% vs 3%; P < .001).24 Furthermore, patients in IRIS who achieved CCyR but not MMR after 18 months had an increased risk of progression at 60 months vs patients who achieved MMR at this timepoint.2,24 In ENESTcmr, the strong responses observed with switch to nilotinib, including achievement of MMR in 83% of patients who were not in MMR when they switched to nilotinib, are of particular clinical relevance considering the international recommendations to achieve MMR within 12 months of commencing TKI therapy given the prognostic significance of this reponse.25
Currently, patients with CML are treated with BCR-ABL1 inhibitors indefinitely.25 One potential advantage of the deeper responses achieved with switch to nilotinib in ENESTcmr is the possibility for TFR, which has drawn increasing interest from both patients and physicians. Criteria for patients to stop treatment in ongoing studies primarily require confirmed, sustained (≥2 years) reduction in BCR-ABL1 levels (MR4.5 or undetectable BCR-ABL1).26,27 Approximately 10% of patients treated with frontline imatinib achieve these levels of response by 2 years.4,26,28,29 In a recent analysis of 423 patients with newly diagnosed CML treated with imatinib, 37% achieved stable MR4.5 by 8 years.5 Both ENESTnd6,7 and ENESTcmr have demonstrated that nilotinib is more effective than imatinib in inducing deeper levels of response, including MR4.5 and undetectable BCR-ABL1. As a result, frontline therapy or a switch to nilotinib may lead to an increase in the number of patients able to benefit from TFR therapeutic strategies. Furthermore, the high degree of overlap between patients who achieved MR4.5 and undetectable BCR-ABL1 in ENESTcmr supports MR4.5 as a more readily standardized criterion for TFR eligibility. The possibility for patients to pursue operational cure of their disease is a strong consideration that must be weighed against the potential for AEs that may occur during the period that patients are treated with nilotinib.
Patients in ENESTcmr were generally younger than patients reported in the general CML population in the United States.30 In addition, the study population consisted of a lower proportion of female patients compared with the general CML population. This is notable because in an analysis of 423 patients in 4 trials treated with frontline imatinib, only the achievement of BCR-ABL1 reduction at 3 months and female sex were independently predictive of achieving stable MR4.5, an important entry criterion for TFR trials.5 Because the baseline characteristics were well balanced between arms, we think it unlikely that these factors substantially affected the results.
The results of the ENESTcmr study indicate that patients with CML-CP with persistent MRD on imatinib who switched to nilotinib experienced deeper confirmed molecular responses compared with those who remained on imatinib, and these responses were stable. Patients in the nilotinib arm also had fewer events, including loss of response, compared with patients in the imatinib arm. In addition to constituting eligibility criteria for TFR studies, these deeper molecular responses have been associated with improved long-term survival outcomes.11,-13 Longer follow-up in ENESTcmr will provide additional information on the long-term efficacy and safety of switching to nilotinib vs remaining on imatinib.
Presented at the 53rd, 54th, and 55th annual meetings of the American Society of Hematology (San Diego, CA, December 10-13, 2011; Atlanta, GA, December 8-11, 2012; and New Orleans, LA, December 7-10, 2013), the 2012 and 2013 annual meetings of the American Society of Clinical Oncology (Chicago, IL, June 1-5, 2012, and May 31 to June 4, 2013), and at the 17th and 18th congresses of the European Hematology Association (Amsterdam, The Netherlands, June 14-17, 2012, and Stockholm, Sweden, June 13-16, 2013).
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
The authors thank Sandip Acharya, John Reynolds, Mary Anne Cox, and Lisa Williams for their input and support with this manuscript. The authors also thank Erinn Goldman, PhD, and Nicole Parker, PhD, for medical editorial assistance with this manuscript.
Financial support for medical editorial assistance was provided by Novartis Pharmaceuticals.
Authorship
Contribution: T.P.H., J.H.L., N.S., D.P., T.S., and B.L. were responsible for the study concept and design; J.H.L., N.S., R.P., N.C.D.C., P.E.D.L., S.B., L.C., T.S., and B.L. collected and assembled data; T.P.H., J.H.L., N.S., F.C., R.P., P.E.D.L., A.P.S., F.-X.M., D.R., S.B., D.P., T.S., and B.L. analyzed and interpreted data; A.P.S., F.-X.M., and B.L. provided study materials or patients; and all authors have drafted and approved the manuscript.
Conflict-of-interest disclosure: T.P.H. acted as a consultant for and received honoraria from Novartis, Bristol-Myers Squibb, and ARIAD and received research funding from Novartis, Bristol-Myers Squibb, and CSL. J.H.L. acted as a consultant for and received research funding from Novartis and received honoraria from Novartis, Bristol-Myers Squibb, Pfizer, TEVA, and ARIAD. N.S. received honoraria, research funding, and other remuneration from Novartis. A.P.S. acted as a consultant for Novartis, Bristol-Myers Squibb, and Pfizer and received honoraria from Novartis and Bristol-Myers Squibb. F-X.M. acted as a consultant for and received honoraria from Novartis and Bristol-Myers Squibb. D.R. received honoraria from Novartis, Bristol-Myers Squibb, TEVA, Pfizer, and ARIAD. S.B. acted as a consultant for and received honoraria and research funding from Novartis, Bristol-Myers Squibb, and ARIAD. D.P., L.C., and T.S. are Novartis Pharmaceuticals employees and stockholders. B.L. acted as a consultant for and received honoraria and research funding from Novartis Canada. The remaining authors declare no competing financial interests.
Correspondence: Timothy P. Hughes, SA Pathology and South Australian Health and Medical Research Institute, University of Adelaide, PO Box 14 Rundle Mall, Frome Rd, Adelaide, SA, 5000, Australia; e-mail: timothy.hughes@health.sa.gov.au.