Key Points
A motif in the immunoglobulin domains of LILRB2 is critical to the multimerized Angptl2 binding and signaling activation.
Immobilized anti-LILRB2 supports ex vivo expansion of human cord blood HSCs.
Abstract
A better understanding of the interaction between extrinsic factors and surface receptors on stem cells will greatly benefit stem cell research and applications. Recently, we showed that several angiopoietin-like proteins (Angptls) bind and activate the immune inhibitory receptor human leukocyte immunoglobulin (Ig)-like receptor B2 (LILRB2) to support ex vivo expansion of hematopoietic stem cells (HSCs) and leukemia development. However, the molecular basis for the interaction between Angptls and LILRB2 was unclear. Here, we demonstrate that Angptl2 expressed in mammalian cells forms high-molecular-weight species and that ligand multimerization is required for activation of LILRB2 for downstream signaling. A novel motif in the first and fourth Ig domains of LILRB2 was identified that is necessary for the receptor to be bound and activated by Angptl2. The binding of Angptl2 to LILRB2 is more potent than and not completely overlapped with the binding of another ligand, HLA-G. Immobilized anti-LILRB2 antibodies induce a more potent activation of LILRB2 than Angptl2, and we developed a serum-free culture containing defined cytokines and immobilized anti-LILRB2 that supports a net expansion of repopulating human cord blood HSCs. Our elucidation of the mode of Angptl binding to LILRB2 enabled the development of a new approach for ex vivo expansion of human HSCs.
Introduction
Hematopoietic stem cell (HSC) transplantation represents an important therapy for hematologic disorders.1 In transplantation, high doses of HSCs are needed to achieve rapid and sustained engraftment that is critical for the patient’s survival and recovery; this is especially true when cord blood HSCs are used.2,3 Although a number of groups have made progress toward efficient ex vivo expansion of HSCs,4-11 significant improvements in the efficacy and reproducibility of this technology are needed before it can be widely used.
Our group has shown that several angiopoietin-like proteins (Angptls) support the activity of HSCs in vitro and in vivo.6,12-14 Angptls are a family of 7 secreted glycoproteins that share sequence homology with angiopoietins, which are important modulators of angiogenesis.15,16 Each Angptl contains an N-terminal coiled-coil (CC) domain and a C-terminal fibrinogen-like (FBN) domain. These proteins are expressed by many types of cells including those from endocrine organs, liver, fat, muscle, and heart,15 as well as the bone marrow HSC niche cells including endothelium and adipocytes.12,15 Expression of Angptls is induced by hypoxia,15 and these proteins clearly play important roles in lipid metabolism, angiogenesis, and inflammation.17 Numerous studies indicate that Angptls, including Angptl2, Angptl4, and Angptl6, support cancer development.18-20 We and others showed that several Angptls inhibit differentiation and promote repopulation of HSCs in vitro and in vivo.6,12,14
Until recently, Angptls were considered “orphan ligands” as no receptors were known. In 2012, we identified human leukocyte immunoglobulin (Ig)-like receptor B2 (LILRB2) and its mouse ortholog paired Ig-like receptor (PirB) as receptors for several Angptls.21 These receptors contain immunoreceptor tyrosine-based inhibitory motifs (ITIM) in their intracellular domains and are classified as inhibitory receptors because ITIM motifs can recruit phosphatases SHP-1, SHP-2, or Src homology 2–containing inositol 5′ phosphatase to negatively regulate cell activation.22,23 To our surprise, we found that LILRB2 and PirB are expressed by human and mouse HSCs, respectively, and support their ex vivo expansion.21 We further demonstrated that the binding of Angptls to LILRB2/PirB induces activation of SHP-2 and Ca2+/calmodulin-dependent kinase, types of factors known to be critical for supporting the activity of HSCs.24,25 We also showed that LILRB2 and PirB are required for leukemia development as they inhibit differentiation and promote self-renewal of leukemic progenitors.21
An important question is how Angptl binding leads to the activation of LILRB2. In this study, we investigated the molecular basis for the interaction between Angptls and LILRB2. We demonstrate that mammalian-expressed Angptl2 exists as a high-molecular-weight (HMW) species, which is needed for activation of LILRB2 and subsequent downstream signaling. We further identified a novel motif in the first and fourth Ig domains of LILRB2 that is critical to the Angptl2 binding. Moreover, we showed that the binding of Angptl2 to LILRB2 is more potent and not completely overlapped with the binding of another ligand, HLA-G. Based on the new understanding of the Angptl/LILRB2 interaction, we developed a serum-free culture containing defined cytokines and immobilized anti-LILRB2 antibodies that supports a stable and reproducible ex vivo expansion of repopulating human cord blood HSCs.
Methods
Chimeric receptor reporter cells
The chimeric receptors consisting of individual or all Ig domains or their mutants of the extracellular domain of LILRB2 and the transmembrane and cytoplasmic domains of activating paired immunoglobulin-like receptor β (PILRβ) were infected into mouse T-cell hybridoma cells carrying a nuclear factor of activated T cells (NFAT) green fluorescent protein (GFP) reporter gene and DAP12 by using a retrovirus vector. Amino acids (aa) 24 to 458 of hLILRB2 were used to construct the full-length LILRB2 chimeric reporter. The amino acid sequences for the individual Ig domains or Ig combinations are Ig1 (aa 24-119), Ig2 (aa 120-219), Ig3 (aa 221-320), Ig4 (aa 321-458), Ig1+2 (aa 24-219), and Ig3+4 (aa 221-458). These chimeric LILRB2-PILRβ receptor reporter cells (5 × 104/well) were incubated with ligands for indicated time, and GFP was analyzed by flow cytometry. Purified Angptl2-FLAG by M2 resin or Angptl2 in conditioned medium collected from transfected 293T cells was used as indicated. For experiments using coated wells, indicated bacterially expressed glutathione S-transferase (GST)-Angptl2, anti-LILRB2 polyclonal antibody (pAb; #BAF2078, R&D Systems), anti-LILRB2 monoclonal antibody (mAb; #16-5149-85, eBioscience), or control antibody was precoated on 96-well plates for 3 hours at 37°C unless otherwise indicated. When antibodies were crosslinked, 10 µg/mL pAb was incubated with 10 µg/mL streptavidin at 4°C overnight.
Details of mice, plasmids, proteins, gel electrophoresis, fast protein liquid chromatography (FPLC), cell culture, transplantation, flow cytometry, retrovirus infection, and confocal microscopy are included in the supplemental Methods available at the Blood Web site. All animal experiments were performed with the approval of the UT Southwestern Committee on Animal Care.
Results
Multimerized Angptl2 activates LILRB2
Because there is no definitive downstream reporter for LILRB-mediated signaling, we employed a stable reporter cell system to test whether Angptl2 can bind to and activate LILRB2. In this chimeric receptor reporter system, the extracellular domain (ECD) of LILRB2 is fused with transmembrane/intracellular domains of PILRβ that associates with the adaptor protein DAP12 containing an immunoreceptor tyrosine-based activation motif. When the chimeric receptor is activated by ligand binding to the ECD of LILRB2, ZAP70 or Syk kinase is recruited to the immunoreceptor tyrosine-based activation motif of the adaptor DAP12 and activates NFATs to promote GFP expression driven by the NFAT-responsive promoter (Figure 1A). The establishment of this reporter was inspired by a LILRB1 reporter system,26,27 and it serves as a surrogate and sensitive system to enable us to study the signaling-induction abilities of different forms of Angptl2 (including soluble, immobilized, monomeric, and oligomeric) and to screen additional agonists and antagonists.
HMW Angptl2 activates LILRB2 signaling. (A) Schematic of the chimeric LILRB2 receptor reporter system. (B) Representative flow cytometric profiles and summary showing that the Angptl2-conditioned medium stimulates GFP induction in the LILRB2 chimeric reporter system. The condition media of empty-vector–transfected HEK-293T cells was used as control. (C) Secreted Angplt2 and HLA-G-ECD in condition medium detected by anti-FLAG antibody in western blotting (left). Representative flow cytometric plots showing that Angptl2 binds to LILRB2 expressed on HEK293T cells better than the same amount of HLA-G-ECD (right). (D) The full-length (FL), CC, and FBN domains obtained from conditioned medium showed distinctive migration in reducing and nonreducing sodium dodecyl sulfate polyacrylamide gel electrophoresis as determined by immunoblotting with anti-M2 Flag antibody. Protein extracted from equivalent amounts of condition media of empty-vector–transfected HEK293T cells was used as control. (E) GST-human Angptl2 purified from bacterial expression system by GST was immediately fractionated through gel-filtration FPLC. The molecular weight was determined by the peaks of apoferritin (443 kDa), amylase (200 kDa), alcohol dehydrogenase (150 kDa), albumin (66 kDa), carbonic anhydrase (29 kDa), and cytochrome c (12.4 kDa), respectively. (F) Equivalent amounts of indicated fractionated samples in FPLC were loaded on 10% native gel. Aggregated, monomeric, and cleaved GST-Angptl2 were visualized by silver staining. (G) Indicated FPLC fractionated samples were examined by western blotting using anti-M2 Flag antibody. The FLAG in cleaved GST-Angptl2 fragments (fraction 8; Figure 1G) could not be detected by western blotting. (H) Chimeric LILRB2 receptor reporter cells were treated with coated or soluble fraction 5 proteins for 48 hours. In coated wells, 5 µg/ml GST-Angptl2 from fraction 5 was precoated onto wells of a 96-well plate for 3 hours at 37°C. An equivalent amount of FPLC buffer was used as control. n.s. indicates not significant; ****P < .0001. Ctrl, control; KD, kilodalton.
HMW Angptl2 activates LILRB2 signaling. (A) Schematic of the chimeric LILRB2 receptor reporter system. (B) Representative flow cytometric profiles and summary showing that the Angptl2-conditioned medium stimulates GFP induction in the LILRB2 chimeric reporter system. The condition media of empty-vector–transfected HEK-293T cells was used as control. (C) Secreted Angplt2 and HLA-G-ECD in condition medium detected by anti-FLAG antibody in western blotting (left). Representative flow cytometric plots showing that Angptl2 binds to LILRB2 expressed on HEK293T cells better than the same amount of HLA-G-ECD (right). (D) The full-length (FL), CC, and FBN domains obtained from conditioned medium showed distinctive migration in reducing and nonreducing sodium dodecyl sulfate polyacrylamide gel electrophoresis as determined by immunoblotting with anti-M2 Flag antibody. Protein extracted from equivalent amounts of condition media of empty-vector–transfected HEK293T cells was used as control. (E) GST-human Angptl2 purified from bacterial expression system by GST was immediately fractionated through gel-filtration FPLC. The molecular weight was determined by the peaks of apoferritin (443 kDa), amylase (200 kDa), alcohol dehydrogenase (150 kDa), albumin (66 kDa), carbonic anhydrase (29 kDa), and cytochrome c (12.4 kDa), respectively. (F) Equivalent amounts of indicated fractionated samples in FPLC were loaded on 10% native gel. Aggregated, monomeric, and cleaved GST-Angptl2 were visualized by silver staining. (G) Indicated FPLC fractionated samples were examined by western blotting using anti-M2 Flag antibody. The FLAG in cleaved GST-Angptl2 fragments (fraction 8; Figure 1G) could not be detected by western blotting. (H) Chimeric LILRB2 receptor reporter cells were treated with coated or soluble fraction 5 proteins for 48 hours. In coated wells, 5 µg/ml GST-Angptl2 from fraction 5 was precoated onto wells of a 96-well plate for 3 hours at 37°C. An equivalent amount of FPLC buffer was used as control. n.s. indicates not significant; ****P < .0001. Ctrl, control; KD, kilodalton.
To test whether Angptl2 expressed in mammalian cells can activate signaling through LILRB2, we incubated the LILRB2 reporter cells with conditioned medium collected from 293T cells transfected with a plasmid designed to express Angptl2 (2 μg/mL) (schematic in supplemental Figure 1). Conditioned medium from mock-transfected 293T cells served as the control. After 24 hours, Angptl2-treated LILRB2 reporter cells induced a significantly greater GFP expression than the control cells (18.95% ± 0.95% vs 5.34% ± 1.19%; Figure 1B). We also measured the potential binding/activation of LILRB2 by the immobilized HLA-G using the same LILRB2 reporter cells and were unable to detect the GFP activation by as much as 130 µg/mL HLA-G (supplemental Figure 2). This suggests that Angptl2 is capable of binding and activating LILRB2, with a significantly greater ability than HLA-G. In parallel, we compared the binding of Angptl2/LILRB2 with HLA-G/LILRB2 using flow cytometry analysis. To this end, we constructed secretable HLA-G-ECD expression vector with the same signal peptide as Angptl2 expression vector (supplemental Figure 1) and collected the same amount of soluble HLA-G-ECD and Angptl2 for binding to LILRB2 expressed on the surface of transiently transfected 293T cells. Similar to the result of the chimeric reporter system, Angptl2 binds to LILRB2 with a significantly greater affinity than HLA-G (Figure 1C).
In our characterization of Angptl2 expressed in 293T cells, we compared full-length, CC, and FBN domains of Angptl2 with or without β-mercaptoethanol treatment. β-Mercaptoethanol treatment reduces disulfide bonds that stabilize an HMW form of Angptl2. When analyzed by sodium dodecyl sulfate polyacrylamide gel electrophoresis, full-length and CC domain preparations of Angptl2 ran partially or exclusively, respectively, as an HMW band (>250 kDa), whereas the FBN domain migrated as a 37-kDa band, corresponding to the expected size of the FBN monomer (Figure 1D). The HMW species was likely in a multimerization state. Similarly, multimerized Angptl2 exists in mouse serum and plasma (supplemental Figure 3).
A bacterial expression system enabled us to produce and purified a large amount of GST-tagged Angptl2.21 We purified the monomer GST-Angptl2 by size exclusion chromatography and detected the product in native polyacrylamide gel electrophoresis and western blotting (Figure 1E-G). Because the multimerized ligand can induce the clustering of surface receptor,26,27 and because immobilized ligand may also cluster the receptor, we sought to compare the abilities of the soluble and immobilized monomeric GST-Angptl2 to activate the LILRB2 reporter cells. The monomeric GST-Angptl2 was immobilized on the wells of the tissue culture plate or added into the medium, and LILRB2 reporter cells were added. Only the immobilized monomeric form, and not the soluble monomer, induced GFP expression (20% ± 2.65% vs 3.56% ± 0.98%; Figure 1H). Because the natively multimerized form of Angptl2 and the immobilized monomer Angptl2 activated LILRB2 but the soluble monomeric Angptl2 did not, we conclude that Angptl2 must be multimerized to become an active ligand of LILRB2.
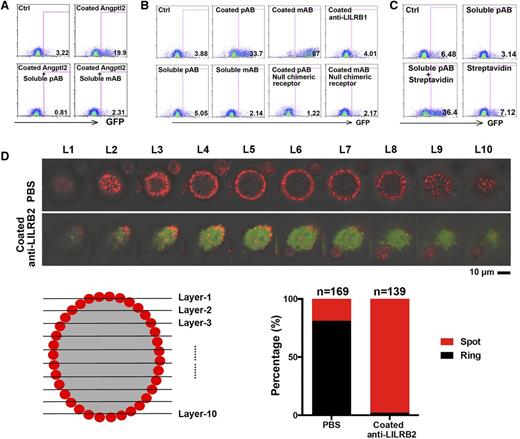
To further study whether a multimerized form of Angptl2 is needed to activate LILRB2, we tested the effects of monoclonal and polyclonal anti-LILRB2 antibodies on the LILRB2 reporter cells. Both soluble monoclonal and polyclonal anti-LILRB2 blocked the activation of LILRB2 by the immobilized Angptl2 (from 19.9% to 0.81 or 2.31%; Figure 2A), supporting the idea that the binding between Angptl2 and LILRB2 is not as potent as that between anti-LILRB2 and LILRB2. Whereas neither the control antibody nor the soluble anti-LILRB2 stimulated GFP expression, both immobilized monoclonal and polyclonal anti-LILRB2 efficiently induced upregulation of GFP (from 3.88% to 33.7% or 87%; Figure 2B). Moreover, crosslinking of biotin-conjugated anti-LILRB2 by streptavidin activated GFP expression (from 3.1% to 36.4%; Figure 2C). Although anti-LILRB2 has a higher binding affinity for LILRB2 than does Angptl2, only immobilized but not soluble antibodies activated LILRB2. To determine if immobilized ligands induce receptor clustering, we examined the LILRB2 localization on the cell surface with or without immobilized monoclonal anti-LILRB2 treatment. Without treatment, the majority of LILRB2 chimeric reporter cells (81.2%) exhibited an even distribution of LILRB2-ECD on the cell membrane, with a “ring”-like shape under confocal microscopy observation (Figure 2D). By contrast, after treatment with immobilized antibodies, the distribution of LILRB2-ECD was changed from a ring-like shape to a “spot”-like shape in 97.8% of signaling activated cells (indicated as GFP induced cells; Figure 2D). These results further support our conclusion that multimerized ligands induce the clustering of the receptor LILRB2 for signaling activation.
Immobilized anti-LILRB2 antibodies activated the chimeric LILRB2 reporter. (A) Representative flow cytometric profiles showing that the GFP induction by immobilized 5 µg/mL Angptl2 was abolished by 5 µg/mL anti-LILRB2 antibody. Chimeric LILRB2 receptor reporter cells were treated with indicated coated Angptl2 with or without soluble anti-LILRB2 pAb or mAb for 48 hours. Phosphate-buffered saline (PBS) was used as control. (B) Representative flow cytometric profiles showing that GFP was induced by immobilized anti-LILRB2 antibodies. Chimeric LILRB2 receptor reporter cells were treated with indicated coated (25 µg/mL in 50 µL PBS) or soluble (5 µg/mL in 250 µL cell culture media) antibodies for 48 hours. The reporter cells not containing chimeric LILRB2 receptor were used as negative control. (C) Representative flow cytometric profiles showing that GFP expression was induced by crosslinked anti-LILRB2 antibodies. Chimeric LILRB2 receptor reporter cells were treated with 10 µg/mL soluble anti-LILRB2 polyclone antibody (pAb) or equivalent crosslinked pAb for 48 hours. Streptavidin alone was used as a negative control. (D) Representative confocal images of LILRB2 chimeric receptor reporter cells with or without coated anti-LILRB2 mAb showing that the distribution of LILRB2 protein on cell plasma membrane. Ten confocal scans from top to bottom of a cell were indicated from layer 1 (L1) to layer 10 (L10). Confocal images of the phase contrast, Cy3 (indicating LILRB2 expression), and GFP (indicating signaling activation) panels were merged. Ctrl, control.
Immobilized anti-LILRB2 antibodies activated the chimeric LILRB2 reporter. (A) Representative flow cytometric profiles showing that the GFP induction by immobilized 5 µg/mL Angptl2 was abolished by 5 µg/mL anti-LILRB2 antibody. Chimeric LILRB2 receptor reporter cells were treated with indicated coated Angptl2 with or without soluble anti-LILRB2 pAb or mAb for 48 hours. Phosphate-buffered saline (PBS) was used as control. (B) Representative flow cytometric profiles showing that GFP was induced by immobilized anti-LILRB2 antibodies. Chimeric LILRB2 receptor reporter cells were treated with indicated coated (25 µg/mL in 50 µL PBS) or soluble (5 µg/mL in 250 µL cell culture media) antibodies for 48 hours. The reporter cells not containing chimeric LILRB2 receptor were used as negative control. (C) Representative flow cytometric profiles showing that GFP expression was induced by crosslinked anti-LILRB2 antibodies. Chimeric LILRB2 receptor reporter cells were treated with 10 µg/mL soluble anti-LILRB2 polyclone antibody (pAb) or equivalent crosslinked pAb for 48 hours. Streptavidin alone was used as a negative control. (D) Representative confocal images of LILRB2 chimeric receptor reporter cells with or without coated anti-LILRB2 mAb showing that the distribution of LILRB2 protein on cell plasma membrane. Ten confocal scans from top to bottom of a cell were indicated from layer 1 (L1) to layer 10 (L10). Confocal images of the phase contrast, Cy3 (indicating LILRB2 expression), and GFP (indicating signaling activation) panels were merged. Ctrl, control.
A motif in Ig domains of LILRB2 is critical for the effects of Angptl2 in binding and receptor activation
LILRB2 contains 4 extracellular Ig-like domains.22 We hypothesized that one or more of these Ig domains bind to Angptl2. To test this hypothesis, we generated a number of domain- and site-specific mutations of LILRB2 for the flow cytometry–based cell-surface ligand binding assay and chimeric reporter assay. To start with, we screened the Angptl2 binding abilities of a number of domain mutations of LILRB2. Although individual Ig domains of LILRB2 do not bind to Angptl2, Ig1 and Ig2 in combination and Ig3 and Ig4 in combination displayed about 50% and 10%, respectively, of the maximal binding between the full-length LILRB2 and Angptl2 (Figure 3A-B and supplemental Figure 4). This suggests that the major Angptl2 binding site resides in Ig1 and Ig2 of LILRB2 and that Ig3 and Ig4 facilitate ligand binding.
Ig domains 1 and 4 in LILRB2 are critical for Angptl2 binding and signal activation. (A) Representative flow cytometry plots showing Angptl2 binding to full-length, individual Ig domain, Ig1+2, or Ig3+4 of LILRB2 that were expressed on 293T cells; n = 3. (B) Summary of data from panel A. (C) Summary of Angptl2 binding abilities of wild-type (WT) and mutant LILRB2. Indicated mutations are described in supplemental Figure 3B. (C) Schematic of the H*G*Y*C motifs in Ig1 and Ig4 of LILRB2. (D) Summary of Angptl2 binding abilities of WT and mutant Ig1+2 LILRB2. (E) Representative flow cytometry plots showing Angptl2 binding to Ig1+2 and mutant LILRB2. (F) Representative flow cytometry plots showing Angptl2 binding to WT and mutant LILRB2. (G) Comparison of Angptl2, Angptl5, and HLA-G binding abilities of WT and mutant LILRB2. MHC-S indicates HLA-G binding sites; MHC-S1, R59A/Y61A; MHC-S2, W90A/D200A/N202A/Y205A; MHC-S1+2, R59A/Y61A/W90A/D200A/N202A/Y205A. (H) Summary of Angptl2-induced activation of the chimeric receptor reporter system by individual Ig domains, Ig1+2, or Ig3+4 of LILRB2. Indicated reporter cells were treated with 5 µg/mL coated GST-Angptl2 or polyclonal or monoclonal anti-LILRB2 antibodies. At least 3 independent experiments gave the similar results. (I) Summary of Angptl2-induced activation of the chimeric receptor reporter system by WT or mutant LILRB2. Reporter cells were treated with 10 µg/mL coated GST-Angptl2 or polyclonal or monoclonal anti-LILRB2 antibodies. At least 3 independent experiments were performed that gave the similar results. NC, negative control cells that do not express the chimeric receptors.
Ig domains 1 and 4 in LILRB2 are critical for Angptl2 binding and signal activation. (A) Representative flow cytometry plots showing Angptl2 binding to full-length, individual Ig domain, Ig1+2, or Ig3+4 of LILRB2 that were expressed on 293T cells; n = 3. (B) Summary of data from panel A. (C) Summary of Angptl2 binding abilities of wild-type (WT) and mutant LILRB2. Indicated mutations are described in supplemental Figure 3B. (C) Schematic of the H*G*Y*C motifs in Ig1 and Ig4 of LILRB2. (D) Summary of Angptl2 binding abilities of WT and mutant Ig1+2 LILRB2. (E) Representative flow cytometry plots showing Angptl2 binding to Ig1+2 and mutant LILRB2. (F) Representative flow cytometry plots showing Angptl2 binding to WT and mutant LILRB2. (G) Comparison of Angptl2, Angptl5, and HLA-G binding abilities of WT and mutant LILRB2. MHC-S indicates HLA-G binding sites; MHC-S1, R59A/Y61A; MHC-S2, W90A/D200A/N202A/Y205A; MHC-S1+2, R59A/Y61A/W90A/D200A/N202A/Y205A. (H) Summary of Angptl2-induced activation of the chimeric receptor reporter system by individual Ig domains, Ig1+2, or Ig3+4 of LILRB2. Indicated reporter cells were treated with 5 µg/mL coated GST-Angptl2 or polyclonal or monoclonal anti-LILRB2 antibodies. At least 3 independent experiments gave the similar results. (I) Summary of Angptl2-induced activation of the chimeric receptor reporter system by WT or mutant LILRB2. Reporter cells were treated with 10 µg/mL coated GST-Angptl2 or polyclonal or monoclonal anti-LILRB2 antibodies. At least 3 independent experiments were performed that gave the similar results. NC, negative control cells that do not express the chimeric receptors.
Next, we designed a series of site-specific mutations in amino acids potentially critical to the binding of ligand to LILRB2 based on the structures of LILRB2 binding to HLA-G or other ligands (Protein Data Bank [PDB] structure 2GW5 and 2DYP for Ig1-Ig2 domains, and structure 4LLA for Ig3-Ig4 domains). For this type of Ig structure, the binding interface is possibly located at flexible and variable loops rather than rigid and conserved β-sheets. We therefore proposed the plausible Angptl2/LILRB2 interfaces on individual Ig domains (supplemental Figure 5). Considering the overall geometry of 4 Ig domains is highly flexible and each one of it is not essential, we identified a number of large and hydrophobic residues for mutagenesis study (supplemental Figure 5A-B). However, these mutant LILRB2s do not significantly decrease Angptl2 binding (supplemental Figure 5C). These results suggest that Angptl2 may have somewhat different binding pockets in LILRB2 than HLA-G (see below for additional data).
We then combined bioinformatical analysis and mutagenesis study and found that AA92-100 in Ig1 is critical for Angptl2 binding. The Ig1+2 fragment with mutations in this region (Mut-8aa) did not bind to Angptl2, and Angptl2 binding of the full-length LILRB2 with the same mutation was decreased by more than 50% relative to the wild-type protein (Figure 3C-D). Further experiments showed that single mutations in G94, R95, or Y96 each decreased the Angptl2 binding of full-length LILRB2 by half (Figure 3E), indicating these 3 amino acids are essential for Angptl2 binding in Ig1. A similar motif, AA390-396, exists in Ig4 (Figure 3C). A single mutation in Y394 decreased Angptl2 binding of full-length LILRB2 by about 30% (Figure 3E). Furthermore, when combined with Y96A, G392D or Y394A mutations totally abrogated the Angptl2 binding of full-length LILRB2 (Figure 3E-F). Therefore, these results suggest that Ig2 helps Ig1 to form the major Angptl2 binding site, Ig4 further facilitates Ig1+2 binding, and the H*G*Y*C motifs in Ig1 and Ig4 are critical for LILRB2 to bind Angptl2.
To further investigate whether Angptl2, Angptl5, and HLA-G bind to the same regions in LILRB2, we compared the bindings of these ligands with mutant LILRB2. HLA-G binds to a number of mutant LILRB2 including H92S, T93A, G94D, R95E, Q99R, G392D, T393E, and HLA-G binding site 1 (MHC-S1) (from the structures by Shiroishi et al28,29 ) with lower affinity than Angptl2. Angptl5 binds to mutant LILRB2 generally more similar to Angptl2 than to HLA-G (Figure 3G and supplemental Figure 1B). Together with the result in supplemental Figure 5C, our data suggest that the binding of Angptl2 or HLA-G to LILB2 is partially but not completely overlapped.
In addition to the flow cytometry–based binding analysis, we measured the activation of various LILRB2 mutants treated with immobilized antibodies or Angptl2 in the chimeric reporter system. We found that only Ig1 and Ig2 domains in combination could bind and be activated by ligands (Figure 3H). Moreover, a single mutation in Y96 led to a dramatic decrease of GFP induction, and the combined mutations of Y96A with either G392D or Y394A totally abrogated the GFP induction by immobilized Angptl2 (Figure 3I). Therefore, our results in using the chimeric reporter system confirmed that the H*G*Y*C motifs in Ig1 and Ig4 are essential for LILRB2 to bind Angptl2. Furthermore, they suggest that the indicated motifs are critical for LILRB2 activation.
To identify the sites in Angptls that bind to LILRB2, we compared the binding of the full-length, the CC domain, and the FBN domain of Angptl2 with LILRB2. The full-length protein, but not the CC or the FBN domain of Angptl2, bound to 293T cells that expressed LILRB2.21 The full-length protein and the CC domain (but not the FBN domain) of Angptl2 bound to LILRB2+ human cord blood mononuclear cells (supplemental Figure 6). The full-length Angptl2, the FBN domain, and a high concentration of soluble CC domain were able to activate LILRB2 reporter cells (supplemental Figure 7). We speculate that the actual concentration of the CC domain coated on the plastic dish might be lower than that of the soluble CC domain, and therefore this immobilized CC domain was not sufficiently high to activate the LILRB2 chimeric reporter. These results suggest that both the CC and FBN domains of Angptl2 are needed for optimal binding and activation of LILRB2.
Anti-LILRB2 antibodies support ex vivo expansion of human cord blood HSCs
Although numerous conditions have been used for expansion of HSCs in culture, the optimal mixture of growth factors and cytokines to allow expansion sufficient for clinically applicability has not yet been determined.3-11,30,31 Previously, we identified Angptls as growth factors for HSC expansion.12,14,32 However, because Angptls are large glycosylated proteins that are readily degraded and form aggregates, they are difficult to express and easily lose activity after purification. Molecules with enhanced stability and higher activities that mimic the effects of the Angptls would lead to the development of a more efficient HSC expansion system. Based on our finding that immobilized antibody to LILRB2 mimicked Angptl2-stimulated receptor signaling (Figure 2), we sought to test whether immobilized anti-LILRB2 antibody would support ex vivo expansion of human cord blood HSCs.
We first compared culture of human cord blood CD133+ cells in STF medium, medium with soluble anti-LILRB2, and medium with immobilized either polyclonal or monoclonal anti-LILRB2 antibody. We plated 1 × 104 cryopreserved human cord blood CD133+ cells in indicated medium; after 10 days of culture, the total numbers of cells were determined. More expansion resulted from culture with immobilized pAb or mAb than either in STF medium or in STF medium containing soluble antibodies (230% and 125% of the STF sample value, respectively; Figure 4A-B). It is of note that the levels of CD34+CD90+ cells that may be enriched in cultured HSCs were similar in these conditions (Figure 4C).
Immobilized anti-LILRB2 antibodies promote the proliferation of human cord blood cells in vitro. (A) Human CD133+ umbilical cord blood cells were cultured in STF medium with or without the same amounts of coated (25 µg/mL in 50 µL PBS) or soluble (5 µg/mL in 250 µL StemSpan media) anti-LILRB2 pAb. Total cell expansion was assessed after 10 days of culture (n = 3). (B) Human CD133+ umbilical cord blood cells were cultured in STF medium with or without the same amounts of coated (25 µg/mL in 50 µL PBS) or soluble (5 µg/mL in 250 µL StemSpan media) anti-LILRB2 mAb. Total cell expansion was assessed after 10 days of culture (n = 3). (C) Representative flow cytometric profiles showing the frequency of CD34+CD90+ cells after 10 days of culture. (D-E) Expansion of 250 input equivalent human cord blood CD133+ cells treated with or without anti-LILRB2 pAb (D) or mAb (E) were serially plated in CFU medium. Total CFUs were counted after 7 days in culture. *P < .05; ***P < .001. Ctrl, control; n.s., not significant.
Immobilized anti-LILRB2 antibodies promote the proliferation of human cord blood cells in vitro. (A) Human CD133+ umbilical cord blood cells were cultured in STF medium with or without the same amounts of coated (25 µg/mL in 50 µL PBS) or soluble (5 µg/mL in 250 µL StemSpan media) anti-LILRB2 pAb. Total cell expansion was assessed after 10 days of culture (n = 3). (B) Human CD133+ umbilical cord blood cells were cultured in STF medium with or without the same amounts of coated (25 µg/mL in 50 µL PBS) or soluble (5 µg/mL in 250 µL StemSpan media) anti-LILRB2 mAb. Total cell expansion was assessed after 10 days of culture (n = 3). (C) Representative flow cytometric profiles showing the frequency of CD34+CD90+ cells after 10 days of culture. (D-E) Expansion of 250 input equivalent human cord blood CD133+ cells treated with or without anti-LILRB2 pAb (D) or mAb (E) were serially plated in CFU medium. Total CFUs were counted after 7 days in culture. *P < .05; ***P < .001. Ctrl, control; n.s., not significant.
We next performed colony-forming assays. Concordant with the cell-growth results, samples of cells treated with the immobilized antibodies had significantly increased colony-forming units (CFUs) in both primary and secondary colony-forming assays than the STF sample or the soluble antibody sample (Figure 4D-E). In particular, the immobilized pAb- and mAb-treated samples had more than 3-fold and 1.6-fold of CFUs, respectively, than the STF samples in the secondary replating.
Finally, we transplanted cells cultured in these same conditions into sublethally irradiated NSG mice (1 × 104 input equivalent cells per mouse). CD133+ cells cultured with immobilized anti-LILRB2 pAb had a significantly greater average chimerism in peripheral blood and bone marrow than the counterparts without antibody treatment or cells treated with soluble antibody at the posttransplant time points we analyzed (3-36 weeks; Figure 5A-B). Figure 5C shows human hematopoietic engraftment at 36 weeks in representative mice that were transplanted with differently cultured human cord blood CD133+ cells. Mice that were transplanted with cells cultured in immobilized antibody displayed a much higher engraftment of total hematopoietic (CD45/71+) (52.48% ± 5.41% vs 23.13% ± 7.93%; Figure 5B), myeloid (CD15/66b+) (3.29% ± 0.41% vs 1.16% ± 0.38%; Figure 5D), B-lymphoid (CD34−CD19/20+) (27.43% ± 5.15% vs 8.52% ± 2.58%; Figure 5E), and primitive (CD34+) (0.93% ± 0.24% vs 0.26% ± 0.10%; Figure 5F) human cells than mice transplanted with STF cultured cells or cells cultured with soluble antibody (Figure 5B,D-F).
Ex vivo expansion of human cord blood CD133+ cells by anti-LILRB2 pAb as determined by NSG transplantation. (A) After 10 days of culture in STF medium with or without same amounts of coated (25 µg/mL in 50 µL PBS) or soluble (5 µg/mL in 250 µL StemSpan media) anti-LILRB2 pAb, expansion of 1 × 104 input equivalent human cord blood CD133+ cells were transplanted into NSG mice (n = 8). Engraftment of human cells (human CD45+) in peripheral blood at indicated weeks are shown***P < .001. (B) Engraftment of human CD45/CD71+ in bone marrow of mice described in panel A at 36 weeks. *P < .05; n = 8. (C) Multilineage contribution of cultured human umbilical cord blood CD133+ cells. Shown are representative flow cytometric profiles of bone marrow cells from 1 primary transplanted mouse of each group. Myeloid, CD45/CD71+CD15/CD66b+; lymphoid, CD19/CD20+; hematopoietic stem/progenitor cells, CD19/CD20−CD34+. (D-F) Summary of multilineage contributions from data shown in panel C. *P < .05; **P < .01; n = 8. (G) Engraftment of human CD45+ cells in peripheral blood of secondarily transplanted mice at 3 and 7 weeks posttransplant are shown. **P < .01; n = 3. (H) Engraftments of human cells in bone marrow of secondarily transplanted mice at 8 weeks posttransplant are shown. *P < .05; n = 3. (I) Representative flow cytometric profiles showing multilineage contribution of human umbilical cord blood CD133+ cells in the bone marrow of secondarily transplanted mice at 8 weeks posttransplant. (J-L) Summary of multilineage contributions from data shown in panel I. *P < .05; n = 3. Ctrl, control; n.s., not significant.
Ex vivo expansion of human cord blood CD133+ cells by anti-LILRB2 pAb as determined by NSG transplantation. (A) After 10 days of culture in STF medium with or without same amounts of coated (25 µg/mL in 50 µL PBS) or soluble (5 µg/mL in 250 µL StemSpan media) anti-LILRB2 pAb, expansion of 1 × 104 input equivalent human cord blood CD133+ cells were transplanted into NSG mice (n = 8). Engraftment of human cells (human CD45+) in peripheral blood at indicated weeks are shown***P < .001. (B) Engraftment of human CD45/CD71+ in bone marrow of mice described in panel A at 36 weeks. *P < .05; n = 8. (C) Multilineage contribution of cultured human umbilical cord blood CD133+ cells. Shown are representative flow cytometric profiles of bone marrow cells from 1 primary transplanted mouse of each group. Myeloid, CD45/CD71+CD15/CD66b+; lymphoid, CD19/CD20+; hematopoietic stem/progenitor cells, CD19/CD20−CD34+. (D-F) Summary of multilineage contributions from data shown in panel C. *P < .05; **P < .01; n = 8. (G) Engraftment of human CD45+ cells in peripheral blood of secondarily transplanted mice at 3 and 7 weeks posttransplant are shown. **P < .01; n = 3. (H) Engraftments of human cells in bone marrow of secondarily transplanted mice at 8 weeks posttransplant are shown. *P < .05; n = 3. (I) Representative flow cytometric profiles showing multilineage contribution of human umbilical cord blood CD133+ cells in the bone marrow of secondarily transplanted mice at 8 weeks posttransplant. (J-L) Summary of multilineage contributions from data shown in panel I. *P < .05; n = 3. Ctrl, control; n.s., not significant.
To measure the self-renewal potential of severe combined immunodeficiency–repopulating cells (SRCs) after culture, we collected bone marrow from the primary mice and transplanted them into sublethally irradiated secondary recipients. Engraftment of secondary recipients with cells cultured in STF medium or in soluble antibody was barely detectable. In contrast, the cells cultured with immobilized antibody showed positive engraftment of myeloid, B-lymphoid, and primitive cells after the secondary transplantation (Figure 5G-L). Similar results were obtained from another independent experiment using human cord blood CD34+ cells for culture (supplemental Figure 8).
We also transplanted CD133+ cells cultured with soluble or immobilized anti-LILRB2 mAb into sublethally irradiated NSG mice (1 × 104 input equivalent cells per mouse). In the primary transplantation, engraftment with immobilized monoclonal anti-LILRB2–treated cells was detectable but not significantly different from cells cultured without antibody treatment or with soluble anti-LILRB2 (Figure 6A-F). In the secondary transplantation, however, only cells treated with immobilized anti-LILRB2 showed positive engraftment (Figure 6G-L). Together, our data indicate a net expansion of HSCs during the initial culture period, and we thus conclude that immobilized anti-LILRB2 antibodies support extensive ex vivo expansion of human SRCs.
Ex vivo expansion of human cord blood CD133+ cells by anti-LILRB2 monoclonal antibody in NSG mice as determined by NSG transplantation. (A) After 10 days culture in STF medium with or without same amounts of coated (25 µg/mL in 250 µL PBS) or soluble (5 µg/mL in 250 µL StemSpan media) anti-LILRB2 mAb, 1 × 104 input equivalent human cord blood CD133+ cells were transplanted into NSG mice. Engraftment of human CD45+ in peripheral blood at 3 and 7 weeks are shown. *P < .05; n = 4. (B) Engraftments of human CD45/CD71+ in bone marrow of mice described in panel A at 8 weeks; n = 4. (C) Multilineage contribution of cultured human umbilical cord blood CD133+ cells. Shown are representative flow cytometric profiles of bone marrow cells from 1 primary transplanted mouse of each group. (D-F) Summary of multilineage contributions based on data shown in panel C; n = 4. (G) Engraftment of human CD45+ cells in peripheral blood of secondarily transplanted mice at 3, 7, 10, and 30 weeks. *P < .05; n = 3. (H) Engraftment of human cells in bone marrow of secondarily transplanted mice at 30 weeks; n = 3. (I) Representative flow cytometric profiles showing multilineage contribution of human umbilical cord blood CD133+ cells in the bone marrow of secondarily transplanted mice at 8 weeks posttransplant. (J-L) Summary of multilineage contributions based on data from panel I. *P < .05; n = 3. Ctrl, control; n.s., not significant.
Ex vivo expansion of human cord blood CD133+ cells by anti-LILRB2 monoclonal antibody in NSG mice as determined by NSG transplantation. (A) After 10 days culture in STF medium with or without same amounts of coated (25 µg/mL in 250 µL PBS) or soluble (5 µg/mL in 250 µL StemSpan media) anti-LILRB2 mAb, 1 × 104 input equivalent human cord blood CD133+ cells were transplanted into NSG mice. Engraftment of human CD45+ in peripheral blood at 3 and 7 weeks are shown. *P < .05; n = 4. (B) Engraftments of human CD45/CD71+ in bone marrow of mice described in panel A at 8 weeks; n = 4. (C) Multilineage contribution of cultured human umbilical cord blood CD133+ cells. Shown are representative flow cytometric profiles of bone marrow cells from 1 primary transplanted mouse of each group. (D-F) Summary of multilineage contributions based on data shown in panel C; n = 4. (G) Engraftment of human CD45+ cells in peripheral blood of secondarily transplanted mice at 3, 7, 10, and 30 weeks. *P < .05; n = 3. (H) Engraftment of human cells in bone marrow of secondarily transplanted mice at 30 weeks; n = 3. (I) Representative flow cytometric profiles showing multilineage contribution of human umbilical cord blood CD133+ cells in the bone marrow of secondarily transplanted mice at 8 weeks posttransplant. (J-L) Summary of multilineage contributions based on data from panel I. *P < .05; n = 3. Ctrl, control; n.s., not significant.
We performed a limiting dilution assay to quantitate the SRC frequencies before and after culture. Human cord blood CD133+ cells cultured with immobilized anti-LILRB2 antibody cultured for 10 days had a 112-fold increase in total nucleated cells and a 19-fold increase in CD34+ cells relative to input cells (Figure 7A-B). Cultured cells had greater average chimerism than in peripheral blood (Figure 7C) and bone marrow (Figure 7D). As part of the limiting dilution assay, we measured the engraftment by 700 to 4000 uncultured CD133+ cells and the progenies of these cells after culture. All mice transplanted with the cultured progenies of 4000 CD133+ cells engrafted at a level >1%. Figure 7E shows that the frequency of repopulating cells (CRU) from uncultured CD133+ cells is 1 per 4557 cells (95% confidence interval for mean: 3222 to 6445, n = 24). That is, as calculated from Poisson statistics, injection of an average of 4557 cells from this lot of uncultured human CD133+ cells would be sufficient to repopulate 63% (= 1 − 1/e) of transplanted mice. In contrast, the CRU after culture was 1 per 932 input equivalent cells (95% confidence interval for mean: 689 to 1261, n = 24). There was a 4.9-fold increase in the number of SRCs after they were cultured in STF medium with immobilized anti-LILRB2 polyclonal antibody. These cultured cells had much greater levels of multilineage engraftment than uncultured cells (Figure 7F-I).
Net ex vivo expansion of cultured human umbilical cord blood CD133+ cells as determined by limiting dilution analysis. (A-B) Numbers of total nucleated cells (A) and CD34+ cells (B) before and after culture with 25 µg/mL coated anti-LILRB2 pAb. (C-D) Percentages of donor human CD45+ cells (C) in the peripheral blood at 1 and 2 months and (D) in bone marrow in recipient NSG mice transplanted with uncultured or expanded cells. (E) Net expansion of HSCs as determined by limiting dilution analysis. The numbers of input equivalent cells were used in the calculation. (F-I) Comparisons of multilineage repopulation of HSCs before and after ex vivo expansion. *P < .05; **P < .01; n = 8. MNC, mononuclear cell; n.s., not significant.
Net ex vivo expansion of cultured human umbilical cord blood CD133+ cells as determined by limiting dilution analysis. (A-B) Numbers of total nucleated cells (A) and CD34+ cells (B) before and after culture with 25 µg/mL coated anti-LILRB2 pAb. (C-D) Percentages of donor human CD45+ cells (C) in the peripheral blood at 1 and 2 months and (D) in bone marrow in recipient NSG mice transplanted with uncultured or expanded cells. (E) Net expansion of HSCs as determined by limiting dilution analysis. The numbers of input equivalent cells were used in the calculation. (F-I) Comparisons of multilineage repopulation of HSCs before and after ex vivo expansion. *P < .05; **P < .01; n = 8. MNC, mononuclear cell; n.s., not significant.
Discussion
We previously showed that several Angptls support ex vivo expansion of HSCs,14,32,33 but the mechanism responsible for this activity was unknown. Here, we demonstrated that mammalian-expressed Angptl2 exists as HMW species and that ligand multimerization is required for the activation of LILRB2 for downstream signaling. We further identified motifs in the Ig domains of LILRB2 that are critical for the Angptl2 binding and signaling activation. We showed that the binding of Angptl2 to LILRB2 is greater than and not completely overlapped with the binding of another ligand, HLA-G. In an attempt to identify agonists of LILRB2 that are more potent and stable than Angptl to support ex vivo expansion of human HSCs, we found that immobilized polyclonal anti-LILRB2 supports consistent ex vivo expansion of human cord blood HSCs. This study thus started to uncover the molecular basis for Angptl/LILRB2 interaction. It also provides functional evidence that manipulation of the binding between the ligands and LILRB2 on HSCs supports the repopulating activity of HSCs and demonstrated a novel approach for efficient expansion of HSCs that may find utility in HSC-based cell therapies.
Although the bona fide signaling reporter of LILRB2 is not available, we developed a chimeric receptor surrogate reporter system that can evaluate the ability of a ligand to bind and activate LILRB2. As shown in our study, this reporter cell line can serve as a sensitive system to enable us to compare the signaling-induction abilities of different forms of ligands. This chimeric receptor reporter system will also be useful to screen additional agonists and antagonists of ITIM-containing receptors. We envision that the agonists of ITIM-containing receptors may facilitate stem cell–based regenerative medicine and that the antagonists may serve as inhibitors of cancer development.
We identified the critical factors that contribute to LILRB2 activation by Angptl2. First, we determined that mammalian-expressed Angptl2 forms an HMW species that appears to be important for its binding to the receptor. Angptl2 contains both CC and FBN domains. Based on several pieces of evidence, neither the CC domain nor the FBN domain of Angptl2 alone binds to LILRB2 as potently as the full-length Angptl2. Both the CC domain and full-length LILRB2 exist as HMW species, whereas the FBN domain does not. Concordantly, a previous study showed that the CC domain of Angptl4 mediates multimerization.34 These data suggest that both the CC and FBN domains contribute to the receptor binding and that CC-domain–mediated multimerization significantly enhances the binding of the full-length Angptl2 to LILRB2. Although Angptls are observed as soluble hormones in serum, they can also be enriched on the plasma membrane in vitro (data not shown). In addition to the soluble multimerized form, we speculate that clustered, cell-surface–bound Angptls exist in vivo and activate LILRB2.
We further identified the features of LILRB2 important for Angptl2 binding. Novel H*G*Y*C motifs in the first and fourth Ig domains of LILRB2 are essential to Angptl2 binding. The critical necessity of this motif in Angptl2/LILRB2 binding is supported by the effects of the site-specific mutations at G94, R95, and Y96 in Ig1 that reduced Angptl2 binding by about 50%. Similarly, a single mutation in Y394 in Ig4 decreased Angptl2 binding of full-length LILRB2 by about 40%. In addition to Angptls, LILRB2 is known to have other ligands including various major histocompatibility complex (MHC) class I molecules.29 An important question is whether Angptl and MHC class I molecules bind to LILRB2 in a similar or different manner. As the flow cytometry-based ligand binding assay showed, Angptl2 has a greater binding than HLA-G to LILRB2. This conclusion is supported by the result of our reporter cell assay. In contrast to Angptl2, a much greater dose of the immobilized HLA-G cannot induce GFP expression of the LILRB2 reporter cells. Therefore, Angptl2 is much more potent than HLA-G to activate the LILRB2 chimeric receptor reporter; however, Angptl2 is not efficient enough to compete the binding of HLA-G to LILRB2 (data not shown). Whereas HLA-G binds to the first 2 Ig domains of LILRB2,28 Angptl2 binds to both Ig1 and Ig4 of LILRB2. Interestingly, the H*G*Y*C motif that is critical for Angptl2 binding to LILRB2 is not within either of the 2 MHC binding sites or at the typical interfacial loop region but within the β-sheet structure, as revealed by a crystallographic study.28 Based on our binding and activation results, this motif should be required for the conformational stability of the Ig domains of LILRB2 that is needed for binding and activation by Angptl2. Together, these results suggest that (1) Angptl2 binds and activates LILRB2 with a greater ability than MHC class I and (2) Angptl2 and MHC class I molecules may not directly compete for binding to the same sites, and these 2 types of molecules may be able to act individually or even cooperatively so that the signaling of LILRB2 may be coregulated. Nevertheless, the binding of each of the 2 ligands may somewhat require common conformational alterations. For example, although the mutations of most of the MHC class I binding sites of LILRB2 did not affect Angptl2 binding, the mutation G94D, R95E, or Y394A in LILRB2 that is within the H*G*Y*C motif and decreases Angptl2 binding also lowers HLA-G binding. Interestingly, whereas all LILRBs contain a G*Y*C motif, Angptl2 only binds LILRB2. This suggests that the H*G*Y*C motif is necessary but not sufficient for maintaining the LILRB2 conformation for Angptl2 binding and activation.
Based on our identification of the binding sites of Angptl2 to LILRB2, we developed an improved strategy to use immobilized anti-LILRB2 for ex vivo expansion of human HSCs. Immobilized antibodies bind to the same region of LILRB2 as Angptl2. The serum-free culture system containing defined cytokines and immobilized anti-LILRB2 supports a net expansion of repopulating human cord blood HSCs, as determined by the serial NSG transplantation and limiting dilution analysis. The polyclonal anti-LILRB2 antibody demonstrated a greater ability to support ex vivo expansion of HSCs than monoclonal anti-LILRB2, suggesting the possibility of multiple ligand binding sites in LILRB2 activation. Together, because the anti-LILRB2 polyclonal antibodies are easier to be expressed and purified and more stable than Angptls and, importantly, bind and activate LILRB2 with a greater ability than Angptl2, this system may have greater advantages for use in ex vivo expansion of HSCs. In addition, the existence of the internalization signal YXXphi in LILRB235 suggests that LILRB2 can undergo endocytosis, possibly after ligand binding. The use of immobilized anti-LILRB2 may thus prevent LILRB2 internalization and prolong the receptor activation, further enhancing the ex vivo expansion of HSCs.
The online version of this article contains a data supplement.
There is an Inside Blood Commentary on this article in this issue.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
This study was supported by the National Institutes of Health, National Cancer Institute grant 1R01CA172268, DOD W81XWH-10-1-0429, the Leukemia & Lymphoma Society (awards 260071 and 6024-14), the Robert A. Welch Foundation (grant I-1834), the Gabrielle’s Angel Foundation, the March of Dimes Foundation Award #1-FY14-201 (C.C.Z.), the Shenzhen Bureau of Science Technology and Information (grant JC201005260239A) (Z. L.), and the Program for Professor of Special Appointment (Eastern Scholar) at Shanghai Institutions of Higher Learning and the Pujiang Program (13PJ1405600) (J. Z.).
Authorship
Contribution: M.D., Z.L., J. Z., and C.C.Z. contributed to design, performed experiments, interpreted data, and wrote the manuscript; X.W., X.C., K.H., H. S., Y.L., L.C., Q.W., C.S., N.H., G.F.G., and Y.J. performed experiments; H.A. contributed to the concept and construction of the chimeric reporter stable cell line; and M.D. made modifications to the reporter system.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Junke Zheng, Chongqing South Rd 280, Shanghai 200025, China; e-mail: zhengjunke@sjtu.edu.cn; and Cheng Cheng Zhang, 5323 Harry Hines Blvd, ND5.136E, Dallas, TX 75390-9133; e-mail: alec.zhang@utsouthwestern.edu.
References
Author notes
M.D. and Z.L. contributed equally to this study.