Key Points
Patients with PMF may carry JAK2 (V617F), a CALR exon 9 indel, an MPL exon 10 mutation, or none of these genetic lesions.
The genetic subtypes of PMF differ substantially as regards clinical course, disease progression, and overall survival.
Abstract
We studied the impact of driver mutations of JAK2, CALR, (calreticulin gene) or MPL on clinical course, leukemic transformation, and survival of patients with primary myelofibrosis (PMF). Of the 617 subjects studied, 399 (64.7%) carried JAK2 (V617F), 140 (22.7%) had a CALR exon 9 indel, 25 (4.0%) carried an MPL (W515) mutation, and 53 (8.6%) had nonmutated JAK2, CALR, and MPL (so-called triple-negative PMF). Patients with CALR mutation had a lower risk of developing anemia, thrombocytopenia, and marked leukocytosis compared with other subtypes. They also had a lower risk of thrombosis compared with patients carrying JAK2 (V617F). At the opposite, triple-negative patients had higher incidence of leukemic transformation compared with either CALR-mutant or JAK2-mutant patients. Median overall survival was 17.7 years in CALR-mutant, 9.2 years in JAK2-mutant, 9.1 years in MPL-mutant, and 3.2 years in triple-negative patients. In multivariate analysis corrected for age, CALR-mutant patients had better overall survival than either JAK2-mutant or triple-negative patients. The impact of genetic lesions on survival was independent of current prognostic scoring systems. These observations indicate that driver mutations define distinct disease entities within PMF. Accounting for them is not only relevant to clinical decision-making, but should also be considered in designing clinical trials.
Introduction
Primary myelofibrosis (PMF) is a Philadelphia-negative myeloproliferative neoplasm (MPN) characterized by abnormal proliferation of megakaryocytes, deposition of fibrous connective tissues in the bone marrow, abnormal stem cell trafficking, and extramedullary hematopoiesis (myeloid metaplasia).1,2 In an international study of 1054 patients with PMF, the overall median survival was found to be 5.8 years, but considerable variability was observed.3 This study identified age >65 years, presence of constitutional symptoms, hemoglobin level <10 g/dL, leukocyte count >25 × 109/L, and circulating blast cells 1% or greater as independent predictors of shortened survival at diagnosis. The use of these parameters led to the definition of the International Prognostic Scoring System (IPSS), which identifies 4 prognostic groups with substantially different survival in PMF.3 A subsequent study investigated whether the acquisition of the above factors during follow-up predicted survival of PMF patients, and eventually led to the development of the dynamic IPSS (DIPSS).4
The 2008 World Health Organization (WHO) definition of PMF includes JAK2 (V617F) or MPL (W515) mutations as a major diagnostic criterion that unequivocally proves the clonal nature of the disease.1 However, the genomic landscape of PMF has changed considerably since then.5 In 2013, somatic mutations of CALR, the gene encoding calreticulin, have been found in 20% to 25% of patients with essential thrombocythemia (ET) or PMF.6,7 Like JAK2 and MPL mutations, somatic mutations of CALR behave as driver mutations responsible for the myeloproliferative phenotype.5 Recent studies have also identified subclonal mutations in genes like ASXL1, SRSF2, EZH2, IDH1, and IDH2, which are commonly associated with disease progression and identify PMF patients at high risk for leukemic transformation or premature death.8,9
In the original study on the identification of calreticulin mutations in patients with ET or PMF, a multivariate Cox regression analysis of overall survival (OS) showed that patients with a CALR mutation had a lower risk of death than those with JAK2 (V617F) or an MPL mutation.6 In the current work, we studied a large population of patients with PMF followed at 4 different centers and analyzed the impact of driver mutations of JAK2, CALR, or MPL on clinical course, risk of leukemic transformation, and OS.
Patients and methods
This study was approved by the institutional ethics committee (Comitato di Bioetica, Fondazione Istituto di Ricovero e Cura a Carattere Scientifico [IRCCS] Policlinico, San Matteo, Pavia, Italy), and by the institutional review boards of the remaining centers. The procedures followed were in accordance with the Helsinki Declaration of 1975, as revised in 2000, and samples were obtained after patients had provided written informed consent.
Study population and definitions
Inclusion in the current study required the availability of demographic, clinical, and hematologic data at diagnosis (age, evaluation of constitutional symptoms, hemoglobin level, white blood cell count, and percentage of blasts in peripheral blood) that allow calculation of IPSS, and at least 1 DNA sample to assess mutation status of the 3 driver genes: JAK2, MPL, and CALR. A total of 617 patients with PMF were recruited from 4 centers: 187 from the Department of Hematology Oncology and 171 from the Center for the Study of Myelofibrosis (Pavia, Italy), 163 from the University Hospital (Florence, Italy), and 96 from the Hospital Clínic (Barcelona, Spain).
Diagnoses of PMF and that of leukemic transformation (blast phase) were performed in accordance with the 2008 WHO criteria.1 For the assessment of bone marrow fibrosis, paraffin sections were stained with Gomori silver impregnation technique, and fibrosis was assessed semiquantitatively following the European consensus guidelines.10 Thrombotic events were defined as described in detail by the CYTO-PV Collaborative Group.11 IPSS and DIPSS risks were estimated as previously described.3,4
JAK2, CALR, and MPL mutation analysis
Granulocyte JAK2 (V617F) mutation status and mutant allele burden were assessed using a quantitative polymerase chain reaction–based allelic discrimination assay on a Rotor-Gene 6000 real-time analyzer (Qiagen), as previously described.12-14 Patients without JAK2 (V617F) were evaluated for MPL exon 10 mutations using a high-resolution melt assay or Sanger sequencing.15,16 Patients with nonmutated JAK2 and MPL were studied for CALR exon 9 mutations as reported in our original article6 or by Sanger sequencing, as described elsewhere.16
Statistical analysis
Numerical variables have been summarized by their median and range, and categorical variables by count and relative frequency (%) of each category. Comparisons of quantitative variables between groups of patients were carried out by the nonparametric Wilcoxon rank-sum test. The Wilcoxon signed-rank test was applied to compare measures of quantitative variables repeated in different phases of the disease. Association between categorical variables (2-way tables) was tested by the Fisher exact test. The cumulative incidence of anemia, thrombocytopenia, marked leukocytosis, thrombotic events, and leukemic transformation was estimated with a competing risk approach, considering death for any cause as a competing event.17 The comparison of cumulative incidence curves in different groups of patients was carried out using the Pepe-Mori test,18 whereas the effect of quantitative covariates was estimated by applying the Fine-Gray regression model.19 OS was estimated using the Kaplan-Meier product limit method, and survival curves of different subgroups (JAK2-mutant, CALR-mutant, MPL-mutant, and triple-negative patients) were compared by the log-rank test. Multivariate analysis of OS was carried out by Cox regression. Prognostic scores were analyzed as both a fixed and a time-dependent covariate. The Akaike information criterion (AIC) was applied to compare quality of models.20 This criterion provides a measure of the relative goodness of fit of a statistical model and a means for comparison among models, a lower AIC value indicating a better tradeoff between fit and complexity.
All P values were considered statistically significant when <.05 (2-tailed). Statistical analyses were performed using Stata 12.1 (StataCorp LP) software.
Results
Presenting hematologic and clinical features of PMF patients according to JAK2, CALR, and MPL mutation status
Of the 617 patients studied, 399 (64.7%) carried JAK2 (V617F), 140 (22.7%) a CALR exon 9 indel, 25 (4.0%) an MPL (W515) mutation, and 53 (8.6%) had nonmutated JAK2, MPL, and CALR (ie, triple-negative subjects). Clinical phenotypes at diagnosis are reported in Table 1. CALR-mutant patients were significantly younger, had lower leukocyte count, higher platelet count, and lower IPSS risk. On the opposite, triple-negative patients were older, had lower hemoglobin level, lower platelet count, and higher IPSS risk (P values in Table 1).
Demographic and clinical features at diagnosis of 617 patients with PMF subdivided according to their genotype (JAK2, CALR, and MPL mutation status)
| . | JAK2 (V617F)-mutant patients . | CALR-mutant patients . | MPL-mutant patients . | Patients with nonmutated JAK2, CALR, and MPL (triple-negative subjects) . | P . |
|---|---|---|---|---|---|
| No. (%) | 399 (64.7%) | 140 (22.7%) | 25 (4.0%) | 53 (8.6%) | |
| Sex (male/female) | 266/133 | 77/63 | 17/8 | 34/19 | .101 |
| Age at onset, median (range), y | 63 (18-91) | 50 (26-83) | 64 (31-84) | 67 (31-88) | <.001 |
| Hemoglobin, median (range), g/dL | 12 (3-19.6) | 11.7 (7.1-15.9) | 11 (6.5-15) | 9.9 (5-19) | <.001 |
| WBC count, median (range), ×109/L | 10 (1.6-106.2) | 8.2 (2.2-45) | 8.4 (2.1-20.3) | 8.4 (2.4-90.8) | .002 |
| PLT count, median (range), ×109/L | 310 (25-1963) | 509 (46-1563) | 307 (53-958) | 175 (19-3279) | <.001 |
| Circulating blasts, median (range), % | 0 (0-20) | 0 (0-10) | 0 (0-4) | 0 (0-16) | <.001 |
| Lactate dehydrogenase, median (range), mU/mL | 553 (149-3440) | 692 (203-3610) | 580 (183-2291) | 531 (160-3173) | .208 |
| Circulating CD34+ cells, median (range), ×106/L | 16.2 (0.8-1190) | 34.2 (1.7-1902) | 100 (6.3-506.3) | 45.3 (1.6-485.5) | .022 |
| IPSS risk group, % | |||||
| Low | 31 | 51 | 28 | 10 | <.001 |
| Intermediate 1 | 31 | 23 | 36 | 26 | |
| Intermediate 2 | 22 | 18 | 24 | 17 | |
| High | 16 | 8 | 12 | 47 |
| . | JAK2 (V617F)-mutant patients . | CALR-mutant patients . | MPL-mutant patients . | Patients with nonmutated JAK2, CALR, and MPL (triple-negative subjects) . | P . |
|---|---|---|---|---|---|
| No. (%) | 399 (64.7%) | 140 (22.7%) | 25 (4.0%) | 53 (8.6%) | |
| Sex (male/female) | 266/133 | 77/63 | 17/8 | 34/19 | .101 |
| Age at onset, median (range), y | 63 (18-91) | 50 (26-83) | 64 (31-84) | 67 (31-88) | <.001 |
| Hemoglobin, median (range), g/dL | 12 (3-19.6) | 11.7 (7.1-15.9) | 11 (6.5-15) | 9.9 (5-19) | <.001 |
| WBC count, median (range), ×109/L | 10 (1.6-106.2) | 8.2 (2.2-45) | 8.4 (2.1-20.3) | 8.4 (2.4-90.8) | .002 |
| PLT count, median (range), ×109/L | 310 (25-1963) | 509 (46-1563) | 307 (53-958) | 175 (19-3279) | <.001 |
| Circulating blasts, median (range), % | 0 (0-20) | 0 (0-10) | 0 (0-4) | 0 (0-16) | <.001 |
| Lactate dehydrogenase, median (range), mU/mL | 553 (149-3440) | 692 (203-3610) | 580 (183-2291) | 531 (160-3173) | .208 |
| Circulating CD34+ cells, median (range), ×106/L | 16.2 (0.8-1190) | 34.2 (1.7-1902) | 100 (6.3-506.3) | 45.3 (1.6-485.5) | .022 |
| IPSS risk group, % | |||||
| Low | 31 | 51 | 28 | 10 | <.001 |
| Intermediate 1 | 31 | 23 | 36 | 26 | |
| Intermediate 2 | 22 | 18 | 24 | 17 | |
| High | 16 | 8 | 12 | 47 |
Different types of CALR exon 9 mutations and their frequency
Within 140 CALR-mutant patients, 101 (72%) had the 52-bp deletion (L367fs*46, type 1 mutation), 22 (16%) had the 5-bp insertion (K385fs*47, type 2 mutation), and 17 (12%) carried other less frequent indels. The frequency of type 1 mutation in patients with PMF was significantly higher than that previously reported by us in patients with ET16 (72% vs 46%, P < .001).
Risk of development of anemia, thrombocytopenia, marked leukocytosis, and large splenomegaly during the clinical course according to JAK2, CALR, and MPL mutation status
We estimated the time to development of anemia (here defined as a hemoglobin level <10 g/dL), thrombocytopenia (platelet [PLT] count <100 × 109/L), and marked leukocytosis (white blood cell [WBC] count >25 × 109/L) using a competing risk approach.
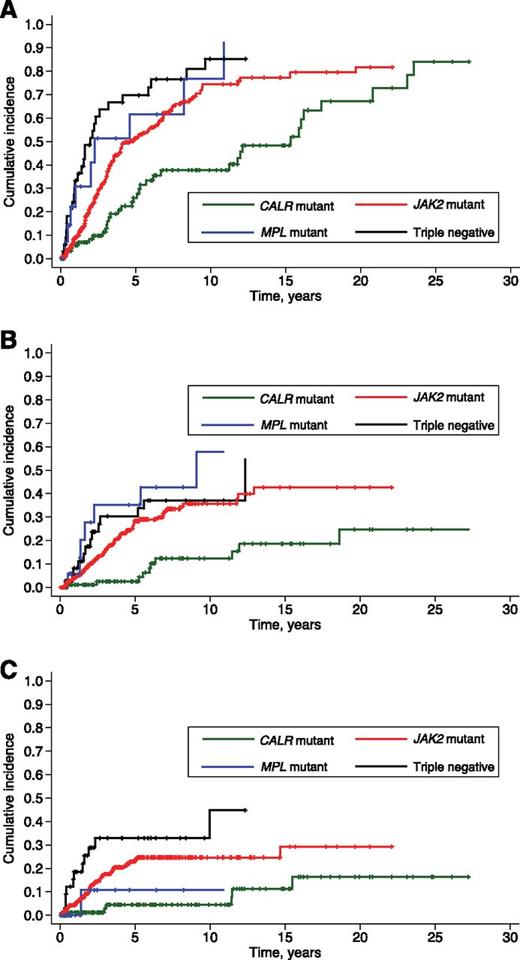
As shown in Figure 1A, CALR-mutant patients had a lower cumulative incidence of anemia compared with JAK2-mutant (P < .001), MPL-mutant (P = .004), and triple-negative patients (P < .001). On the opposite, triple-negative patients were more likely to develop anemia compared with either CALR-mutant (P < .001) or JAK2-mutant subjects (P = .013).
Cumulative incidence of anemia, thrombocytopenia, and marked leukocytosis in PMF patients stratified according to their driver mutation. The thresholds for hemoglobin level and WBC count are those of the IPSS,3 whereas that for PLT count is the lower limit of normal range. Cumulative incidences were estimated with a competing risk approach, considering death for any cause as a competing event. Vertical tick marks indicate right-censored patients. (A) Cumulative incidence of anemia (hemoglobin <10 g/dL). CALR-mutant patients had a lower incidence of anemia compared with the remaining patients (maximum P value equal to .004). (B) Cumulative incidence of thrombocytopenia (PLT count <100 × 109/L). The cumulative incidence of thrombocytopenia was significantly lower in CALR-mutant patients compared with the remaining ones (P < .001 in all comparisons). (C) Cumulative incidence of marked leukocytosis (WBC count >25 × 109/L). The cumulative incidence of marked leukocytosis was significantly lower in CALR-mutant patients compared with JAK2-mutant (P = .004) or triple-negative patients (P < .001).
Cumulative incidence of anemia, thrombocytopenia, and marked leukocytosis in PMF patients stratified according to their driver mutation. The thresholds for hemoglobin level and WBC count are those of the IPSS,3 whereas that for PLT count is the lower limit of normal range. Cumulative incidences were estimated with a competing risk approach, considering death for any cause as a competing event. Vertical tick marks indicate right-censored patients. (A) Cumulative incidence of anemia (hemoglobin <10 g/dL). CALR-mutant patients had a lower incidence of anemia compared with the remaining patients (maximum P value equal to .004). (B) Cumulative incidence of thrombocytopenia (PLT count <100 × 109/L). The cumulative incidence of thrombocytopenia was significantly lower in CALR-mutant patients compared with the remaining ones (P < .001 in all comparisons). (C) Cumulative incidence of marked leukocytosis (WBC count >25 × 109/L). The cumulative incidence of marked leukocytosis was significantly lower in CALR-mutant patients compared with JAK2-mutant (P = .004) or triple-negative patients (P < .001).
The cumulative incidence of thrombocytopenia was significantly lower in CALR-mutant patients compared with the remaining ones (P = .001), whereas no significant difference was observed between triple-negative and JAK2-mutant (P = .292) or MPL-mutant patients (P = .627) (Figure 1B).
The cumulative incidence of marked leukocytosis was significantly lower in CALR-mutant patients compared with JAK2-mutant (P = .004) or triple-negative patients (P < .001). These latter showed a particularly high risk at 3 years (>30%, Figure 1C).
Large splenomegaly was defined as a spleen tip extending >10 cm from left costal margin, as previously reported.21 Time to large splenomegaly could be estimated only in patients from the Pavia Center for the Study of Myelofibrosis. CALR-mutant patients had a significantly longer large-splenomegaly-free survival compared with the remaining patients with PMF (P < .001, data not shown).
Cumulative risk of thrombosis according to JAK2, CALR, and MPL mutation status
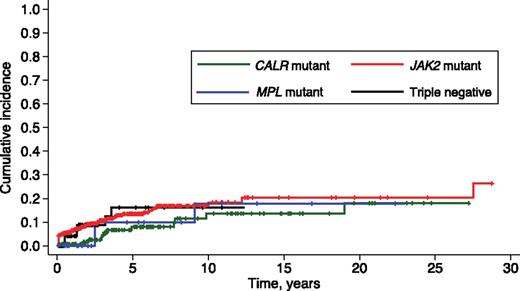
Thrombotic complications occurred in 76 of 617 patients (12%) with PMF. The 10-year cumulative incidence of thrombosis was 18.3% (95% confidence interval [CI], 13.3-24.1) in patients carrying JAK2 (V617F), 13.6% (95% CI, 6.9-22.7) in those with CALR mutation, 17.9% (95% CI, 4.1-39.6) in those with MPL mutation, and 16.2% (95% CI, 6.3-30.1) in triple-negative subjects (Figure 2). Patients carrying a CALR mutation had a lower risk of thrombosis than those carrying JAK2 (V617F) (P = .021), whereas no significant difference was observed by comparing the other genotypic subgroups.
Cumulative incidence of thrombosis in PMF patients stratified according to their driver mutation. Vertical tick marks indicate right-censored patients. JAK2-mutant patients had a higher incidence of thrombosis than those with CALR mutation (P = .021). This difference remained statistically significant after adjusting for age (SHR, 2.19; 95% CI, 1.15-4.18; P = .017), the estimated risk of thrombosis being about 2-fold in JAK2-mutant compared with CALR-mutant patients.
Cumulative incidence of thrombosis in PMF patients stratified according to their driver mutation. Vertical tick marks indicate right-censored patients. JAK2-mutant patients had a higher incidence of thrombosis than those with CALR mutation (P = .021). This difference remained statistically significant after adjusting for age (SHR, 2.19; 95% CI, 1.15-4.18; P = .017), the estimated risk of thrombosis being about 2-fold in JAK2-mutant compared with CALR-mutant patients.
The above difference in risk of thrombosis remained statistically significant after adjusting for age. In fact, when comparing JAK2-mutant and CALR-mutant patients, the subdistribution hazard ratio (SHR) was 2.19 (95% CI, 1.15-4.18, P = .017), indicating that the risk of thrombosis was about twofold in the former. When taking into account the type of CALR mutation, both patients with type 1 and those with type 2 mutation had a lower risk of thrombosis compared with patients carrying JAK2 (V617F) (P = .042 and P = .021, respectively).
Progression to blast phase according to JAK2, CALR, and MPL mutation status
Seventy-four of 617 patients (12%) progressed to blast phase (leukemic transformation), including 43 of 399 subjects (11%) with JAK2 mutation, 17 of 140 (12%) with CALR mutation, 3 of 25 (12%) with MPL mutation, and 11 of 53 triple-negative subjects (21%).
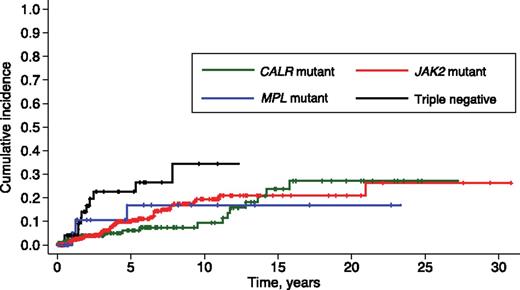
The 10-year cumulative incidence of leukemic transformation was 19.4% (95% CI, 13.9-25.6) in JAK2-mutant, 9.4% (95% CI, 4.3-16.8) in CALR-mutant, 16.9% (95% CI, 4.1-37.1) in MPL-mutant, and 34.4% (95% CI, 16.8-52.8) in triple-negative subjects (Figure 3). These latter patients showed a higher incidence of leukemic transformation compared with both CALR-mutant (P = .016) and JAK2-mutated patients (P = .043). After adjusting for age, the risk of leukemic transformation remained higher in triple-negative patients compared with JAK2-mutant patients (P = .04), whereas the significance of the difference between triple-negative and CALR-mutant patients was borderline (P = .052).
Cumulative incidence of leukemic transformation in PMF patients stratified according to their driver mutation. Vertical tick marks indicate right-censored patients. Triple-negative patients had higher incidence of leukemic transformation compared with both CALR-mutant and JAK2-mutant patients (maximum P value equal to .043).
Cumulative incidence of leukemic transformation in PMF patients stratified according to their driver mutation. Vertical tick marks indicate right-censored patients. Triple-negative patients had higher incidence of leukemic transformation compared with both CALR-mutant and JAK2-mutant patients (maximum P value equal to .043).
OS according to JAK2, CALR, and MPL mutation status
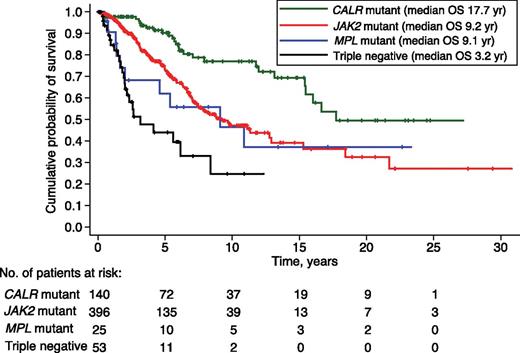
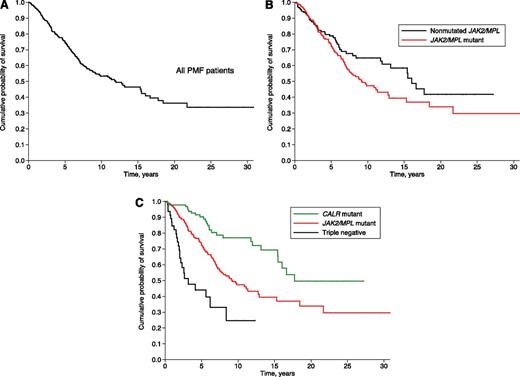
The median follow-up of the study population was 3.5 years (range, 0.1-30.8 years). Death occurred in 176 patients (28.5%), including 115 of 399 patients with JAK2 (V617F) (29%), 27 of 140 with a CALR exon 9 indel (19%), 10 of 25 (40%) with an MPL (W515) mutation, and 24 of 53 triple-negative patients (45%).
Median OS was 17.7 years in CALR-mutant, 9.2 years in JAK2-mutant, 9.1 years in MPL-mutant, and 3.2 years in triple-negative patients, as shown in Figure 4. In univariate analysis, CALR-mutant patients had a better OS than JAK2-mutant (hazard ratio [HR] 2.3, P < .001), MPL-mutant (HR 2.6, P = .009), and triple-negative patients (HR 6.2, P < .001). In a multivariate analysis corrected for age, CALR-mutant patients maintained a better OS compared with either JAK2-mutant (P = .019) or triple-negative patients (P < .001).
Kaplan-Meier analysis of survival of PMF patients stratified according to their driver mutation. Vertical tick marks indicate right-censored patients. In univariate analysis, CALR-mutant patients had a better OS than JAK2-mutant (HR 2.3, P < .001), MPL-mutant (HR 2.6, P = .009), and triple-negative patients (HR 6.2, P < .001). Three JAK2-mutant patients had short follow-up and were not included in the analysis.
Kaplan-Meier analysis of survival of PMF patients stratified according to their driver mutation. Vertical tick marks indicate right-censored patients. In univariate analysis, CALR-mutant patients had a better OS than JAK2-mutant (HR 2.3, P < .001), MPL-mutant (HR 2.6, P = .009), and triple-negative patients (HR 6.2, P < .001). Three JAK2-mutant patients had short follow-up and were not included in the analysis.
When considering the type of CALR mutation, patients carrying a type 1 CALR mutation had a better OS compared with patients carrying JAK2 (V617F) (P < .001). No difference in OS was observed between patients with type 1 and those with type 2 CALR mutation (P = .235), and between patients with type 2 CALR mutation and those with JAK2 (V617F) (P = .311). These results were confirmed after adjusting for time-dependent DIPSS, with a better OS of patients with type 1 CALR mutation compared with those with JAK2 (V617F) (HR 2.01, P = .04), and no difference between patients with type 1 and those with type 2 CALR mutation, as well as between patients with type 2 CALR mutation and those with JAK2 (V617F).
Relative contribution of JAK2, CALR, and MPL mutation status to OS as predicted by IPSS or DIPSS
The impact of the driver mutations on OS was independent of IPSS at diagnosis and of time-dependent DIPSS (maximum P value equal to .033).
To further examine the impact of driver mutations on the clinical course of the disease, we subdivided PMF patients into 2 subgroups according to their IPSS risk: the “lower-risk” subgroup included patients with low or intermediate-1 IPSS risk, whereas the “higher-risk” subgroup included patients with intermediate-2 or high IPSS risk. Within “lower-risk” subjects (Figure 5A), CALR-mutant patients had a better OS compared with either JAK2 (V617F)-mutant (P = .011) or triple-negative patients (P < .001). Within “higher-risk” subjects (Figure 5B), CALR-mutant patients had a better OS compared with all the remaining genetic subgroups (P = .023 compared with JAK2-mutant, P = .003 compared with MPL-mutant, and P = .001 compared with triple-negative patients).
Kaplan-Meier analysis of survival of PMF patients stratified according to their driver mutation and subdivided according to their IPSS risk. Vertical tick marks indicate right-censored patients. (A) “Lower” IPSS risk subgroup, including patients with low or intermediate-1 IPSS risk: CALR-mutant patients had longer survival compared with either JAK2 (V617F)-mutant (P = .011) or triple-negative patients (P < .001). (B) “Higher risk” subgroup, including patients with intermediate-2 or high IPSS risk: CALR-mutant patients had longer survival compared with the remaining genetic subgroups (maximum P value equal to .023).
Kaplan-Meier analysis of survival of PMF patients stratified according to their driver mutation and subdivided according to their IPSS risk. Vertical tick marks indicate right-censored patients. (A) “Lower” IPSS risk subgroup, including patients with low or intermediate-1 IPSS risk: CALR-mutant patients had longer survival compared with either JAK2 (V617F)-mutant (P = .011) or triple-negative patients (P < .001). (B) “Higher risk” subgroup, including patients with intermediate-2 or high IPSS risk: CALR-mutant patients had longer survival compared with the remaining genetic subgroups (maximum P value equal to .023).
Evidence that accounting for JAK2, CALR, and MPL mutation status improves the risk stratification provided by IPSS
We performed a multivariate Cox proportional hazard regression considering type of mutation (CALR, JAK2, MPL, or none of the previous mutations) and each single variable included in the IPSS score at diagnosis (age >65 years, hemoglobin <10 g/dL, WBC count >25 × 109/L, peripheral blood blasts ≥1%, and presence of constitutional symptoms). As shown in Table 2, all of these variables retained a significant independent prognostic effect on OS.
Multivariate Cox proportional hazard regression analysis of driver mutations (JAK2, CALR, and MPL) and IPSS variables evaluated as risk factors for survival in patients with PMF
| Covariates . | HR . | 95% CI . | P . |
|---|---|---|---|
| Driver mutation (JAK2, CALR, and MPL mutation status) | |||
| CALR exon 9 indel* | 1 | ||
| JAK2 (V617F) | 1.9 | 1.2-3.0 | .004 |
| MPL exon 10 mutation | 2.7 | 1.3-5.6 | .009 |
| Nonmutated JAK2, CALR, and MPL | 2.6 | 1.4-4.6 | .002 |
| IPSS variables | |||
| Age >65 y | 3.5 | 2.5-4.9 | <.001 |
| WBC count >25 × 109/L | 3.4 | 2.2-5.2 | <.001 |
| Hemoglobin <10 g/dL | 2.0 | 1.4-2.9 | <.001 |
| Peripheral blood blasts ≥1% | 2.0 | 1.4-2.8 | <.001 |
| Presence of constitutional symptoms | 1.9 | 1.4-2.6 | <.001 |
| Covariates . | HR . | 95% CI . | P . |
|---|---|---|---|
| Driver mutation (JAK2, CALR, and MPL mutation status) | |||
| CALR exon 9 indel* | 1 | ||
| JAK2 (V617F) | 1.9 | 1.2-3.0 | .004 |
| MPL exon 10 mutation | 2.7 | 1.3-5.6 | .009 |
| Nonmutated JAK2, CALR, and MPL | 2.6 | 1.4-4.6 | .002 |
| IPSS variables | |||
| Age >65 y | 3.5 | 2.5-4.9 | <.001 |
| WBC count >25 × 109/L | 3.4 | 2.2-5.2 | <.001 |
| Hemoglobin <10 g/dL | 2.0 | 1.4-2.9 | <.001 |
| Peripheral blood blasts ≥1% | 2.0 | 1.4-2.8 | <.001 |
| Presence of constitutional symptoms | 1.9 | 1.4-2.6 | <.001 |
Reference category.
Considering the independent significance of driver mutations, we developed a prognostic model that includes JAK2, CALR, and MPL mutation status in addition to the IPSS variables. All factors of Table 2 were therefore included in the new prognostic model, here defined as clinical-molecular prognostic model. We assigned each factor an integer weight according to the corresponding HR in the multivariable-Cox regression (Table 2): weight 1 for presence of constitutional symptoms, peripheral blood blasts ≥1%, hemoglobin <10 g/dL, and presence of JAK2 mutation; weight 2 for MPL mutation or nonmutated JAK2, CALR, and MPL, WBC count >25 × 109/L, and age >65 years.
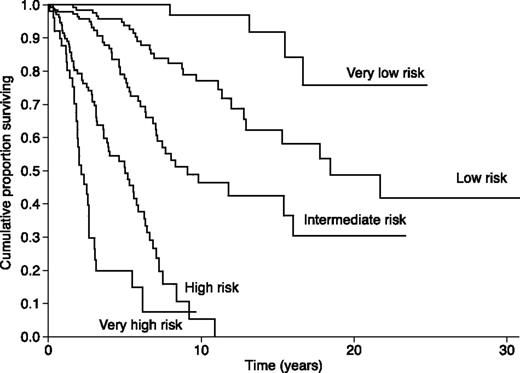
To assess the prognostic impact of the resulting score, we included the score as a continuous covariate in a Cox survival regression model. The HR was 1.83 (95% CI, 1.69-1.99, P < .001), that is, there was a 1.83-fold increase in hazard for a 1-point increase in the sum of weights. To simplify the implementation of the score, we recoded it into 5 broader categories of adequate numerosity by pooling consecutive score values. The resulting risk categories were very low (score = 0), low (score = 1), intermediate (score 2 or 3), high (score 4 or 5), and very high (score ≥6). Of the 617 PMF patients, the clinical-molecular risk was very low in 71 patients, low in 150 patients, intermediate in 202 patients, high in 141 patients, and very high in 53 patients. Kaplan-Meier survival curves corresponding to the 5 score categories were significantly different by log rank-test (Figure 6, P < .001).
Kaplan-Meier analysis of survival of PMF patients stratified according to the risk categories defined by a clinical-molecular prognostic model. This model includes the variables reported in Table 2, that is, IPSS variables plus CALR, JAK2, and MPL mutation status. We assigned each factor an integer weight according to the corresponding HR in the multivariable-Cox regression of Table 2. Scores were then recoded into the 5 risk categories shown in this figure: details are reported in the last section of “Results.” Based on the Akaike information criterion, which compares quality of models, the clinical-molecular model provided a better stratification than the IPSS. This analysis serves as a proof of concept that accounting for driver mutations improves the risk stratification provided by IPSS.
Kaplan-Meier analysis of survival of PMF patients stratified according to the risk categories defined by a clinical-molecular prognostic model. This model includes the variables reported in Table 2, that is, IPSS variables plus CALR, JAK2, and MPL mutation status. We assigned each factor an integer weight according to the corresponding HR in the multivariable-Cox regression of Table 2. Scores were then recoded into the 5 risk categories shown in this figure: details are reported in the last section of “Results.” Based on the Akaike information criterion, which compares quality of models, the clinical-molecular model provided a better stratification than the IPSS. This analysis serves as a proof of concept that accounting for driver mutations improves the risk stratification provided by IPSS.
We then analyzed the categorical clinical-molecular score as a covariate in a Cox survival regression model. Compared with the very-low-risk category, the estimated HR were 4.2 (95% CI, 1.4-12, P = .007) for the low risk, 10.2 (95% CI, 3.6-28.6, P < .001) for the intermediate risk, 37.5 (95% CI, 13.3-105.8, P < .001) for the high risk, and 88.6 (95% CI, 30.3-259, P < .001) for the very-high-risk category.
Finally, we used the Akaike information criterion as a measure of the relative quality of the clinical-molecular prognostic model compared with the IPSS. The former had a lower AIC value (1744.5 vs 1764.3), indicating a better quality for the given set of data.
Discussion
The findings of this study provide a proof of concept that a genetic classification of PMF is not only feasible but also highly relevant to clinical decision-making as regards diagnostic approach and prognostication. In addition, they indicate that PMF genotypes should now be considered also in designing clinical trials on the use of novel drugs for treatment of PMF.
The diversity of PMF subtypes was not fully appreciated as long as the only known mutant genes associated with this condition were JAK2 and MPL, as illustrated in Figure 7. The identification of somatic mutations of calreticulin6,7 has substantially modified our knowledge of PMF. In fact, this identification has split PMF patients with nonmutated JAK2 and MPL (about one-third of all patients with PMF) into 2 distinct subtypes (Figure 7): (1) CALR-mutant PMF, a condition with an indolent clinical course and (2) PMF with nonmutated JAK2, CALR, and MPL, a very aggressive myeloid neoplasm. This advance clearly illustrates the clinical relevance of defining the molecular basis of hematologic malignancies.
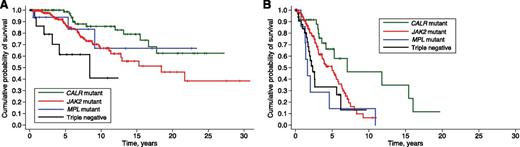
Kaplan-Meier analysis of survival of PMF patients stratified according to their genotype, as it was known in different time periods. (A) OS of the whole population of PMF patients: the genetic basis of MPNs was unknown before 2005, and therefore no genotypic subgroup could be defined. (B) PMF patients stratified according to JAK2 or MPL mutation status: these mutant genes where identified in 2005 and 2006, respectively. (C) PMF patients stratified according to JAK2, CALR, or MPL mutation status: somatic mutations of calreticulin were identified in 2013.
Kaplan-Meier analysis of survival of PMF patients stratified according to their genotype, as it was known in different time periods. (A) OS of the whole population of PMF patients: the genetic basis of MPNs was unknown before 2005, and therefore no genotypic subgroup could be defined. (B) PMF patients stratified according to JAK2 or MPL mutation status: these mutant genes where identified in 2005 and 2006, respectively. (C) PMF patients stratified according to JAK2, CALR, or MPL mutation status: somatic mutations of calreticulin were identified in 2013.
CALR-mutant ET and CALR-mutant PMF have a relatively indolent clinical course compared with the respective JAK2-mutant disorders.6,16,22-25 On the contrary, PMF with nonmutated JAK2, CALR, or MPL has a poor prognosis with a particularly high risk of leukemic transformation, as shown by a study of the Mayo Clinic23 and by the present work. It should be noted, however, that there are major differences in median OS between the same genotypic entities as estimated in the Mayo Clinic vs the present study: 2.5 vs 3.2 years in triple-negative, 4.1 vs 9.1 years in MPL-mutant, 4.3 vs 9.2 years in JAK2-mutant, and 8.2 vs 17.7 years in CALR-mutant patients. At least part of the difference is likely due to the fact that, in the Mayo Clinic study, OS was estimated from the date of diagnosis or first referral, whereas in this study it was always estimated from the date of diagnosis. Analysis from first referral may clearly lead to underestimation of survival.
Ongoing investigations are trying to identify the molecular basis of PMF with nonmutated JAK2, CALR, or MPL, and to better define the distinctive features of these patients. In terms of clinical features, triple-negative PMF is similar to the myelodysplastic syndrome associated with bone marrow fibrosis that we described previously.26 This latter condition is characterized by increased bone marrow cellularity, multilineage dysplasia, severe cytopenia involving high transfusion requirement, unfavorable cytogenetics, and poor survival.26 Compared with PMF patients, those with the myelodysplastic syndrome associated with bone marrow fibrosis have more profound cytopenia and lower circulating CD34-positive cell count.5,27 In addition, they do not carry JAK2 (V617F), which is instead found in two-thirds of PMF patients. Studies are now needed to specifically compare triple-negative PMF with the myelodysplastic syndrome associated with bone marrow fibrosis, but a nonnegligible overlap between the 2 conditions is predictable.27
The remarkable differences in clinical course and outcomes observed among the diverse genetic subtypes suggest that, in spite of similar clinical features (bone marrow fibrosis, abnormal stem cell trafficking, and myeloid metaplasia), the disease biology varies considerably according to the different genetic lesions. Recent observations indicate that the JAK-STAT (Janus kinase–signal transducer and activator of transcription) pathway is activated in all MPNs regardless of founding driver mutations.28 However, the diverse mutations likely involve additional abnormalities in other metabolic pathways; for instance, mutant calreticulin might have peculiar effects on megakaryocyte biology.29
As previously observed in ET,16,22 JAK2 (V617F) appears to be highly thrombophilic also in patients with PMF, indicating that this mutation likely causes thrombosis through multiple mechanisms, including activation of platelets and granulocytes.30,31 On the contrary, despite the fact that calreticulin mutations involve high platelet counts also in PMF, the risk of thrombosis of these patients is relatively low, at least compared with that of JAK2-mutant patients. We did not find major differences between CALR-mutant patients with the 52-bp deletion (type 1 mutation) and those with the 5-bp insertion (type 2 mutation). The only relevant observation was the higher frequency of CALR type 1 mutation in PMF compared with ET, which may suggest a particularly active role of the 52-bp deletion in causing bone marrow fibrosis.
So far, risk stratification in PMF has been essentially based on demographic, clinical, and hematologic parameters.3,4 The findings of this study clearly indicate that the genetic basis of disease and, more specifically, the driver mutation responsible for clinical phenotype is an independent predictor of clinical course and outcomes, and that accounting for that can improve the risk stratification provided by IPSS. Facing a patient with PMF, his or her genetic lesion must now be taken into account carefully because this has an impact on clinical decision-making. As illustrated in Figures 4-5, the prognosis of a triple-negative patient is markedly worse than that of a CALR-mutant patient: in these cases, therapeutic decisions may span from a watchful waiting strategy to allogeneic stem cell transplantation.
The different genetic subtypes of PMF should also be considered in designing clinical trials on the use of novel drugs. As illustrated in Figure 7, at least 3 genetic subgroups should now be taken into account in the interpretation of results: CALR-mutant, JAK2/MPL mutant, and triple-negative patients. It should also be noted that future trials might involve treatments conceived for specific genetic subgroups.
As shown recently by us and by others,9,32 the clinical course of MPNs is profoundly influenced not only by the founding driver mutations but also by subclonal events. The occurrence of subclonal driver mutations might be closely related to the early initiating genetic lesions, as recently observed in myelodysplastic syndromes.33 This latter observation generated the hypothesis of genetic “predestination,” in which early driver mutations dictate future trajectories of subclonal evolution with distinct clinical outcomes.33 The current challenge is to develop a prognostic model that accounts for both clinical and molecular parameters, including relevant subclonal mutations,24 and that in perspective may be useful also for predicting response to different treatments.34 The model reported in Figure 6 was developed with the only objective of showing, as a proof of concept, that genetic data can significantly improve prognostication of PMF. We are not proposing it as clinical tool for several reasons, including the fact that to this purpose it should be validated in an independent patient cohort. As an international collaborative effort, we are currently analyzing clinical, hematologic, and molecular data of a large population of PMF patients with the aim of developing a clinically useful prognostic tool.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
This work was supported by a grant from Associazione Italiana per la Ricerca sul Cancro (AIRC; Milan, Italy), Special Program Molecular Clinical Oncology 5x1000 to AIRC-Gruppo Italiano Malattie Mieloproliferative (AGIMM) project #1005, by grant #GR-2010-2312855 from Italian Ministry of Health (E.R.), by grant #F11J11000250001 from Fondo per Gli Investimenti della Ricerca di Base (FIRB; A.M.V., G.B., and M.C.), and by grant #RD012/0036/0004 from the Instituto de Salud Carlos III, Spanish Ministry of Health (.F.C.).
Authorship
Contribution: M.C., G.B., F.C., C.P., E.R., and A.M.V. designed research; D.P., D.C., M.P., and C. Milanesi performed molecular investigations; P.G., A.M.-T., I.C., L.P., G.R., E.S., M.B., C.C., C. Mannarelli, E.B., C.A., V.R., F.C., G.B., and A.M.V. collected molecular, histologic, and clinical data; C.P. and V.F. performed statistical analyses; and M.C. and E.R. wrote and finalized the manuscript.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
A complete list of the members of the Associazione Italiana per la Ricerca sul Cancro Gruppo Italiano Malattie Mieloproliferative Investigators appears in “Appendix.”
Correspondence: Mario Cazzola, Department of Hematology Oncology, Fondazione IRCCS Policlinico San Matteo, Viale Golgi 19, 27100 Pavia, Italy; e-mail: mario.cazzola@unipv.it.
Appendix
The AGIMM Project (http://www.progettoagimm.it/index_en.shtml) includes the following research units and investigators.
Florence Unit: Alessandro M. Vannucchi (coordinator), Manjola Balliu, Niccolò Bartalucci, Flavia Biamonte, Andrea Bisognin, Costanza Bogani, Stefania Bortoluzzi, Alberto Bosi, Alessandro Coppe, Tiziana Fanelli, Rajmonda Fjerza, Paola Guglielmelli, Idalba Loiacono, Carmela Mannarelli, Roberto Marchioli, Serena Martinelli, Arianna Masciulli, Alessandro Pancrazzi, Chiara Paoli, Lisa Pieri, Giada Rotunno, Claudia Saccoman, Ambra Spolverini, Maria Chiara Susini, and Lorenzo Tozzi.
Pavia Unit 1: Giovanni Barosi (coordinator), Cristina Azzan, Stefania Badalucco, Alessandra Balduini, Elisa Bonetti, Rita Campanelli, Paolo Catarsi, Antonina Maria Isgrò, Maria Letizia Lupo, Umberto Magrini, Margherita Massa, Valentina Poletto, Vittorio Rosti, and Laura Villani.
Pavia Unit 2: Mario Cazzola (coordinator), Ilaria Ambaglio, Paolo Bernasconi, Ilaria Carola Casetti, Silvia Catricalà, Chiara Elena, Elena Fugazza, Anna Gallì, Luca Malcovati, Chiara Milanesi, Cristiana Pascutto, Daniela Pietra, Francesco Ripamonti, Marianna Rossi, and Elisa Rumi.
Milan Unit: Elisabetta Dejana (coordinator), Ferruccio Breviario, Monica Corada, and Benedetta Gaia Erba.
Bergamo Unit: Alessandro Rambaldi (coordinator), Ariel Amaru, Tiziano Barbui, Clara Belotti, Chiara Boroni, Maria Luisa Ferrari, Guido Finazzi, Maria Chiara Finazzi, Josée Golay, Giuseppe Gritti, and Silvia Salmoiraghi.
Torino Unit: Daniela Cilloni (coordinator), Valentina Campia, Sonia Carturan, and Angelo Guerrasio.
Modena Unit: Rossella Manfredini (coordinator), Elisa Bianchi, Monica Montanari, Simona Salati, Enrico Tagliafico, Elena Tenedini, and Roberta Zini.
References
Author notes
E.R., D.P., and C.P. contributed equally to this paper.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal