Key Points
New LVs allow transduction of unstimulated hematopoietic stem cells.
Abstract
Hematopoietic stem cell (HSC)-based gene therapy holds promise for the cure of many diseases. The field is now moving toward the use of lentiviral vectors (LVs) as evidenced by 4 successful clinical trials. These trials used vesicular-stomatitis-virus-G protein (VSV-G)-LVs at high doses combined with strong cytokine-cocktail stimulation to obtain therapeutically relevant transduction levels; however, they might compromise the HSC character. Summarizing all these disadvantages, alternatives to VSV-G-LVs are urgently needed. We generated here high-titer LVs pseudotyped with a baboon retroviral envelope glycoprotein (BaEV-LVs), resistant to human complement. Under mild cytokine prestimulation to preserve the HSC characteristics, a single BaEV-LV application at a low dose, resulted in up to 90% of hCD34+ cell transduction. Even more striking was that these new BaEV-LVs allowed, at low doses, efficient transduction of up to 30% of quiescent hCD34+ cells, whereas high-dose VSV-G-LVs were insufficient. Importantly, reconstitution of NOD/Lt-SCID/γc−/− (NSG) mice with BaEV-LV-transduced hCD34+ cells maintained these high transduction levels in all myeloid and lymphoid lineages, including early progenitors. This transduction pattern was confirmed or even increased in secondary NSG recipient mice. This suggests that BaEV-LVs efficiently transduce true HSCs and could improve HSC-based gene therapy, for which high-level HSC correction is needed for life-long cure.
Introduction
Although the murine leukemia virus (MLV)-based retroviral vectors were successful for hematopoietic stem cell (HSC)-based gene therapy, some clinical trials unfortunately led to severe genotoxic side effects.1-5 Therefore, the lentiviral vectors (LVs), conferring a safer integration profile,6 are gaining interest in the field. Cartier et al reported the successful treatment of patients suffering from X-linked adrenoleukodystrophy by ex vivo correction of autologous HSCs using LVs.7 In the clinical trial of β-globin gene therapy for β-thalassemia, initiated in 2007, the single treated patient is still transfusion independent; however, clonal expansion was detected.8 In 2013, Biffi et al9 and Aiuti et al10 provided very encouraging results for HSC-based gene therapy for the correction of metachromatic leukodystrophy or Wiskott-Aldrich syndrome. Moreover, new trials for X-linked severe combined immunodeficiency and Fanconi anemia, 2 other severe monogenetic defects in children, are being initiated.11
Importantly, for the correction of all these defects in the hematopoietic system, the therapeutic gene must be delivered to cells able both to self-renew and to differentiate into all hematopoietic lineages. Because HSCs meet these criteria, they represent the attractive candidates for gene therapy. Although classical vesicular-stomatitis-virus-G protein (VSV-G)-LVs can transduce nondividing cells, fully quiescent G0 cells are poorly transduced.12-15 Hence, HSCs are poorly permissive for classical VSV-G-LV transduction because 75% of them reside in G0.14 Moreover, we confirmed a very low expression of the VSV receptor, the low density lipid-receptor (LDL-R)16 in unstimulated CD34+ cells, perfectly coinciding with their poor VSV-G-LV-mediated transduction.17 Only early-acting cytokine stimulation of hCD34+ cells, up-regulating the LDL-R, permitted high-level VSV-G-LV transduction.17 However, intense exposure of HSCs to cytokines might affect their homing and extravasation ability and promote differentiation rather than expansion of the HSC pool. 18,19 Moreover, when combining high vector doses with strong cytokine stimulation, the risk for multicopy integration and insertional mutagenesis cannot be neglected.3,4 In an effort to achieve high-level transduction of HSCs under mild transient cytokine stimulation to preserve stem cells fitness,20-22 we engineered LVs displaying stem cell factor (SCF) at their surface, able to efficiently transduce HSCs in vivo.22-24 Therefore, mild cytokine stimulation allowing efficient HSC transduction is an important objective.
Clearly, to reach this goal, alternatives to VSV-G-LVs are urgently needed. As a good alternative, we identified the RD114 feline retrovirus envelope glycoprotein.25 In contrast to VSV-Ggp, RD114gp is resistant to degradation by human complement, an attractive characteristic for in vivo applications.23,25 An RD114 mutant glycoprotein (RDTR) allows efficient pseudotyping of LVs, which confers efficient transduction of hCD34+ HSCs.24-30 RD114 belongs to the betaretroviruses, also including the baboon endogenous retrovirus (BaEV). Both of these viruses use the neutral amino acid (aa) transporter 2 (ASCT-2), present on hCD34+ cells.31-33 Interestingly, BaEV also recognizes another aa transporter, ASCT-1, not recognized by RD114.31,34 The ASCT-1 and -2 receptors, 57% identical in sequence, are differentially expressed in cells and are transporters of an overlapping but nonidentical set of neutral aa.34 Because BaEV uses ASCT-1 in addition to ASCT-2, it demonstrated a broader tropism than RD114.35 Therefore, we hypothesized that LVs displaying the BaEV envelope at their surface might permit an increase in hCD34+ cell transduction.
Here, we generated for the first time LVs that incorporated the BaEVgp at their surface, which efficiently and stably transduced macaque and human hCD34+ cells up to 90% at low vector doses and on mild cytokine stimulation, outperforming by far VSV-G- and RDTR-LVs. Moreover, these new pseudotypes were highly superior over VSV-G and RDTR-LVs for efficient gene transfer into hCD34+ cells in the absence of any exogenous stimuli. Remarkably, these BaEV-LVs transduced up to 30% quiescent hCD34+ cells (vs 5% for VSV-G-LV), with NOD/Lt-SCID/γc−/− (NSG) long-term repopulating capacity of high importance for gene therapy.
Materials and methods
Envelope construction
RDTR is a mutated form of the feline retroviral glycoprotein (gp), RD114, described previously.25 By polymerase chain reaction amplification, the cytoplasmic tail of wild-type (WT) BaEV (BaEVwt) was replaced by MLV-A, resulting in BaEVTR-gp. The BaEVRless-gp was constructed by deletion of the R peptide sequence from BaEVwt-gp (Figure 1A; supplemental Figure 1, available on the Blood Web site).
Modification of the cytoplasmic tails of BaEV glycoproteins allows efficient pseudotyping of lentiviral vectors. (A) Schematic representation of the WT (BaEVwt) and mutant BaEV (BaEVTR, BaEVRless), RDTR- and MLV-A gps. The 17 aa long cytoplasmic domain of the cat and baboon retroviral gps, RD114 and BaEVwt, respectively, was exchanged for one of the MLV-A glycoproteins resulting in the chimeric RDTR- and BaEVTR-gps, respectively. The R peptide of the cytoplasmic tail of BaEVwt was deleted, resulting in the BaEVRLess mutant gps. Amino acid sequences and different domains for these gps are shown in supplemental Figure 1. (B) Titers of the different pseudotyped LVs encoding GFP, obtained by infection of HEK293T cells with serial dilutions of fresh or 100-fold concentrated vector preparations (infectious units/mL). The percentage of GFP+ cells was determined 3 days after infection by FACS (means ± standard deviation [SD], n = 8; ***P < .005). (C) Immunoblots of LV particles displaying the different BaEV-gps at their surface. LVs were purified over a sucrose cushion by ultracentrifugation. The upper part shows staining with antibodies against the surface domain of BaEVwt. The corresponding HIV-1 capsid was revealed to verify equal loading. The positions of the BaEVgps and the HIV-1 capsid (HIV1-CA) are indicated. (D) Pictures show 293T cells 48 hours after transfection with BaEVTR- and BaEVRless-gps.
Modification of the cytoplasmic tails of BaEV glycoproteins allows efficient pseudotyping of lentiviral vectors. (A) Schematic representation of the WT (BaEVwt) and mutant BaEV (BaEVTR, BaEVRless), RDTR- and MLV-A gps. The 17 aa long cytoplasmic domain of the cat and baboon retroviral gps, RD114 and BaEVwt, respectively, was exchanged for one of the MLV-A glycoproteins resulting in the chimeric RDTR- and BaEVTR-gps, respectively. The R peptide of the cytoplasmic tail of BaEVwt was deleted, resulting in the BaEVRLess mutant gps. Amino acid sequences and different domains for these gps are shown in supplemental Figure 1. (B) Titers of the different pseudotyped LVs encoding GFP, obtained by infection of HEK293T cells with serial dilutions of fresh or 100-fold concentrated vector preparations (infectious units/mL). The percentage of GFP+ cells was determined 3 days after infection by FACS (means ± standard deviation [SD], n = 8; ***P < .005). (C) Immunoblots of LV particles displaying the different BaEV-gps at their surface. LVs were purified over a sucrose cushion by ultracentrifugation. The upper part shows staining with antibodies against the surface domain of BaEVwt. The corresponding HIV-1 capsid was revealed to verify equal loading. The positions of the BaEVgps and the HIV-1 capsid (HIV1-CA) are indicated. (D) Pictures show 293T cells 48 hours after transfection with BaEVTR- and BaEVRless-gps.
All chimeric envelope gps were expressed in the phCMV-G expression plasmid.36
Production and titration of LVs
Self-inactivating HIV-1-derived vectors encoding green fluorescent protein (GFP) under the control of a spleen focus foamy virus promoter were generated by transfection of 293T cells.37 For display of RDTR, BaEVTR, and BaEVRless-gps on LVs, 7 μg of the respective envelope gp-encoding plasmids was transfected with gagpol and the self-inactivating (SIN) transfer vector; 3 μg of VSV-Ggp encoding plasmid was used for VSV-G-LV production. Titering of the LVs was performed on 293T cells (supplemental Methods).
Western blot analysis
Western blot analysis was performed as previously described.38 For the detection of the BaEV gps, a goat anti-BaEV polyclonal antibody (ViroMed Biosafety Labs) was used. p24 HIV-1 capsid was detected by anti-HIVp24 antibody to assess equivalent loading of purified vectors.
Sample collection and isolation of CD34+ cells
Cord blood (CB) samples and mobilized blood were collected in sterile tubes containing the anticoagulant citrate dextrose (Sigma-Aldrich) after informed consent and approval was obtained by the institutional review board (Centre International d’Infectiologie, Lyon, France) according to the Declaration of Helsinki. Human and macaque CD34+ cells were isolated as previously described.22,23
Stability of pseudotyped vectors in human/macaque serum
For details, see supplemental Methods.
Transduction of human and macaque CD34+ cells
Human and macaque CD34+ cells were incubated for 14 or 18 to 24 hours (as indicated) in 24-well plates in serum-free medium (CellGro; CellGenix) supplemented with human recombinant cytokines (as indicated): SCF (100 ng/mL), thrombopoietin (TPO) (20 ng/mL), Fms-related tyrosine kinase 3 ligand (Flt3-L) (100 ng/mL), interleukin (IL)-6 (20 ng/mL), and IL-3 (20 ng/mL) (Preprotech); 5 × 104 prestimulated CD34+ cells were transduced in 48-well plates with concentrated LVs at indicated multiplicities of infection (MOIs) in serum-free medium. Cells were replenished with cytokines every 3 days. Three, 6, and 14 days after transduction, the percentage of GFP+ cells was determined by fluorescence-activated cell sorter (FACS). Alternatively, CD34+ cells were freshly isolated and transduced immediately in the absence of cytokines. Where indicated, transductions were performed on RetroNectin-coated plates according to the manufacturer’s instructions (Takara). For myeloid differentiation of CD34+ cells, see supplemental Methods.
Conditioning and reconstitution of NSG mice
NSG mice used were housed in our animal facility (Plateau de Biologie Expérimentale de la Souris, Lyon, France). Experiments were performed in accordance with the European Union guidelines on approval of the protocols by the local ethical committee (Authorization Agreement C2EA-15: Comité d'Evaluation Commun au Centre Léon Bérard, à l'Animalerie de transit de l'ENS, au PBES et au laboratoire P4, Lyon, France).
Two- to 3-day-old newborn NSG mice were subjected to 1-Gy irradiation, and 2 × 105 transduced prestimulated CB CD34+ cells or 5 × 105 transduced prestimulated mobilized CD34+ cells were injected intrahepatically. Unstimulated freshly isolated CD34+ cells were transduced for 24 hours, and 3 × 105 transduced CD34+ cells were injected into NSG mice as above.
After 12 weeks of reconstitution, extensive cell phenotyping in the different hematopoietic tissues (bone marrow [BM], spleen, thymus, and blood) of these mice was performed (supplemental Methods).
For secondary engraftments, the CD34+ cells were isolated from the BM of primary engrafted mice as above, and 4 to 5 × 105 isolated CD34+ cells were injected into newborn NSG mice following the same procedure and phenotype analysis as above.
DNA extraction and quantitative polymerase chain reaction
For details, see supplemental Methods.
Cell cycle analysis
Cell cycle analysis was performed by staining DNA and RNA with 7-amino-actinomycin-D (7AAD) and pyronin-Y.39
Statistical analysis
Statistical analysis was performed using a paired Student t test.
Results
Mutants of the baboon envelope glycoproteins, BaEVTR and BaEVRLess, efficiently pseudotype LVs
Pseudotyping of LVs with WT BaEV gps (Figure 1A) was not efficient because it resulted in fresh and 100-fold concentrated titers of 6 × 103 and 2 × 105 infectious units (IU)/mL, respectively (Figure 1B). Therefore, a chimeric BaEVTR-gp was engineered using the same strategy used to create the RD114 mutant gp, RDTR, by exchange of its cytoplasmic domain for the the murine leukemia virus gp (MLV-A-gp; Figure 1A).25 A second mutant was engineered by the deletion of the fusion inhibitory R peptide resulting in the BaEVRLess-gp (Figure 1A; supplemental Figure 1). We compared these different mutant BaEV-gps to VSV-G- and RDTR-gps for their ability to pseudotype LVs.
RDTR, BaEVTR, BaEVRless, and VSV-G pseudotyped SIN LVs encoding GFP were generated by transient transfection in 293T cells and concentrated 100-fold. Average concentrated titers of 5 × 107 IU/mL were obtained on 293T cells for BaEVTR- and 2 × 108 IU/mL for BaEVRLess-LVs resulting in a 250- and 1000-fold titer increase, respectively, compared with BAEVwt-LVs (Figure 1B; supplemental Table 1). This was in agreement with increased incorporation of BaEVTR- and BaEVRLess-gps on LV particles compared with BaEVwt-gp (Figure 1C). RDTR- and VSV-G-LVs gave titers of 6 × 107 and 1 × 109 IU/mL as expected (Figure 1B).24,25 Physical particle content was similar for the different pseudotypes (supplemental Table 2). Of note, in contrast to BaEVRLess-gp, which induces huge syncytia formation, BaEVTR-gp did not induce any fusion or toxicity in 293T cells (Figure 1D).
The high titers obtained for the BaEVTR- and BaEVRLess-LVs incited us to evaluate them for hCD34+ early progenitor transduction.
BaEVgp pseudotyped LVs outperform VSV-G- and RDTR-LVs for the transduction of immature hCD34+ cells
HSCs are important gene therapy targets because, on correction, they can reconstitute and correct the entire hematopoietic system.7-10 As depicted in Figure 2A, RDTR-LVs only bind to the hASCT2 receptor, whereas both BaEVTR- and BaEVRLess-LVs (BaEV-LVs) bind additionally to amino acid transporter, hASCT1, expressed on the cell membrane (Figure 2A). We prestimulated hCD34+ cells with multiple cytokines (SCF/TPO/Flt3-L) and transduced them with VSV-G-, RDTR-, BaEVTR-, and BaEVRLess-LVs at a MOI of 10 in the presence or absence of the transduction facilitating agent, RetroNectin. The BaEV-LVs outperformed the other pseudotypes for the transduction of hCD34+ cells in the presence of RetroNectin (Figure 2B). Importantly, under these conditions, up to 90% of the hCD34+ cells were transduced by a single exposure to the BaEVTR- or BaEVRLess-LVs. Because it is difficult to compare different pseudotypes using their infectious titers, we also transduced stimulated CD34+ cells with equivalent amounts of physical particles (HIV p24). Significantly higher transduction of CD34+ cells for BaEVgp-LVs compared with VSV-G-LVs was confirmed (supplemental Figure 2A).
BaEV-LVs are superior for the transduction of human CD34+ cells over VSVG-LVs and RDTR-LVs. (A) Schematic representation of LVs pseudotyped by BaEV-gps (BaEVTR or BaEVRLess) that bind to the amino acid transporters, hASCT1 and hASCT2, on the HSC surface. RDTR-LVs only bind to the hASCT2 receptor. Once the LVs fuse with the cell membrane of HSCs, reverse transcription of the viral RNA into proviral DNA occurs followed by its nuclear import and integration into the host genome. (B) Cord blood hCD34+ cells were prestimulated with SCF, TPO, and Flt3-L for 14 hours and transduced with the indicated LV pseudotypes in the presence and absence of RetroNectin at an MOI of 10. VSV-G-LV transductions were performed at an MOI of 10 or 100. GFP expression of the cells was analyzed 6 days after transduction by FACS (means ± SD, n = 5). **P < .05 when comparing BaEVgps with other pseudotypes in the presence of RetroNectin; ***P < .005 when comparing BaEVgps with or without RetroNectin. (C) Percentage of transduction of HEK293T cells by the indicated LV pseudotypes on their incubation at 37°C in heat complement-inactivated or fresh human sera. Data for 3 different serum donors are shown. The stability of virions was calculated as the relative percentage of infectivity of macaque/human serum-treated viruses vs viruses treated with fetal calf serum set to 100%.
BaEV-LVs are superior for the transduction of human CD34+ cells over VSVG-LVs and RDTR-LVs. (A) Schematic representation of LVs pseudotyped by BaEV-gps (BaEVTR or BaEVRLess) that bind to the amino acid transporters, hASCT1 and hASCT2, on the HSC surface. RDTR-LVs only bind to the hASCT2 receptor. Once the LVs fuse with the cell membrane of HSCs, reverse transcription of the viral RNA into proviral DNA occurs followed by its nuclear import and integration into the host genome. (B) Cord blood hCD34+ cells were prestimulated with SCF, TPO, and Flt3-L for 14 hours and transduced with the indicated LV pseudotypes in the presence and absence of RetroNectin at an MOI of 10. VSV-G-LV transductions were performed at an MOI of 10 or 100. GFP expression of the cells was analyzed 6 days after transduction by FACS (means ± SD, n = 5). **P < .05 when comparing BaEVgps with other pseudotypes in the presence of RetroNectin; ***P < .005 when comparing BaEVgps with or without RetroNectin. (C) Percentage of transduction of HEK293T cells by the indicated LV pseudotypes on their incubation at 37°C in heat complement-inactivated or fresh human sera. Data for 3 different serum donors are shown. The stability of virions was calculated as the relative percentage of infectivity of macaque/human serum-treated viruses vs viruses treated with fetal calf serum set to 100%.
In view of clinical in vivo application, it would be an advantage if the BaEV-LVs resist inactivation by the human complement system. Therefore, transduction efficiencies of LVs incubated in fresh or heat-inactivated human sera were compared. Residual transduction efficiencies obtained are shown in Figure 2C. As expected, the VSV-G-LVs were inactivated by the 3 different sera. In contrast, BaEVTR- and BaEVRLess-LVs were resistant to human complement to the same extent as RDTR-LVs (Figure 2C).25
Of importance for their preclinical evaluation, the BAEV-LVs transduced efficiently cynomolgus and macaque BM hCD34+ cells and resisted inactivation by the macaque complement system (supplemental Figure 3A-B).
Taken together, BaEV-LVs are excellent candidates for hCD34+ cell transduction and are better suited than VSV-G-LVs for in vivo applications.
BaEV-LVs allow high-level transduction of hCD34+ cells on mild cytokine prestimulation and at low vector doses
Strong stimulation with a cytokine cocktail can induce HSC differentiation and loss of self-renewal capacity. Thus, the less the cells are prestimulated, the more they keep their stem cell characteristics.18,19,22,23,40 Therefore, we performed LV transductions of hCD34+ cells using different cytokine prestimulation conditions: (1) stimulation with a single cytokine (rSCF or rTPO); (2) rSCF+rTPO; and (3) rSCF+rTPO+rFlt3-L (Figure 3A). Even the strongest cytokine prestimulation used here was milder than cytokine cocktails currently used in a clinical setting. Remarkably, TPO stimulation is sufficient to transduce hCD34+ cells up to 60% using BaEV-LVs, whereas VSVG-LVs and RDTR-LVs transduced only 8% to 20% of the hCD34+ cells (P < .05). A single SCF prestimulation permitted up to 35% to 50% BaEV-LV-mediated transduction of hCD34+ cells, whereas VSVG-LVs and RDTR-LVs reached maximum transduction levels of 10% (P < .05). A combination of TPO+SCF prestimulation and a single BaEV-LV transduction resulted in up to 60% to 80% hCD34+ cell transduction, whereas RDTR- and VSVG-LVs, at the same MOI, achieved on average 40% and 10% transduction, respectively (P < .01 for BaEV-LVs vs VSV-G-LVs). The triple cytokine cocktail (TPO+SCF+Flt-3L) resulted in a gain of transduction levels for the BaEV-LVs (80-90%), whereas significantly lower transduction was obtained for the other pseudotypes (P < .005; Figure 3A).
BaEV-LVs allow high-level transduction of hCD34+ cells following mild cytokine prestimulation. (A) CB hCD34+ cells were prestimulated overnight (18-24 hours) with SCF, TPO, SCF+TPO, or SCF+TPO+Flt3-L and transduced in the presence of RetroNectin by the indicated LV pseudotypes at an MOI of 10. Analysis for the percentage GFP+ cells was performed 6 days after transduction (means ± SD, n = 4). hCD34+ cells were prestimulated with (B) rSCF+rTPO+rFlt3-L or (C) rSCF+rTPO and transduced by the different LV pseudotypes at different MOIs (1, 5, 10, and 20); for VSV-G-LVs, an additional MOI of 100 was applied. Analysis of percentage GFP+ cells was performed 6 days after transduction by FACS (means ± SD, n = 3). (D) CB hCD34+ cells were prestimulated with rSCF+rTPO or rSCF+rTPO+rFlt3-L and subsequently transduced with the indicated LV pseudotypes. An MOI of 10 was applied except for VSV-G LVs (MOI = 100). Three days after transduction, the cells were seeded in myeloid differentiation medium for 14 days. The percentage of GFP+ CFCs is shown for both stimulation conditions (means ± SD, n = 3). (E) Mobilized hCD34+ cells were prestimulated with SCF+TPO+Flt3-L+IL-3 and transduced with the indicated LV pseudotypes at the same vector doses as in D. Myeloid differentiation and transduction analysis was performed as in D. The percentage of GFP+hCD34+ cells and CFCs are shown (means ± SD, n = 3). All transduction were performed in the presence of RetroNectin, and P < .05 when comparing CFC transduction levels of BaEV-LVs with the other pseudotypes.
BaEV-LVs allow high-level transduction of hCD34+ cells following mild cytokine prestimulation. (A) CB hCD34+ cells were prestimulated overnight (18-24 hours) with SCF, TPO, SCF+TPO, or SCF+TPO+Flt3-L and transduced in the presence of RetroNectin by the indicated LV pseudotypes at an MOI of 10. Analysis for the percentage GFP+ cells was performed 6 days after transduction (means ± SD, n = 4). hCD34+ cells were prestimulated with (B) rSCF+rTPO+rFlt3-L or (C) rSCF+rTPO and transduced by the different LV pseudotypes at different MOIs (1, 5, 10, and 20); for VSV-G-LVs, an additional MOI of 100 was applied. Analysis of percentage GFP+ cells was performed 6 days after transduction by FACS (means ± SD, n = 3). (D) CB hCD34+ cells were prestimulated with rSCF+rTPO or rSCF+rTPO+rFlt3-L and subsequently transduced with the indicated LV pseudotypes. An MOI of 10 was applied except for VSV-G LVs (MOI = 100). Three days after transduction, the cells were seeded in myeloid differentiation medium for 14 days. The percentage of GFP+ CFCs is shown for both stimulation conditions (means ± SD, n = 3). (E) Mobilized hCD34+ cells were prestimulated with SCF+TPO+Flt3-L+IL-3 and transduced with the indicated LV pseudotypes at the same vector doses as in D. Myeloid differentiation and transduction analysis was performed as in D. The percentage of GFP+hCD34+ cells and CFCs are shown (means ± SD, n = 3). All transduction were performed in the presence of RetroNectin, and P < .05 when comparing CFC transduction levels of BaEV-LVs with the other pseudotypes.
In gene therapy, insertional mutagenesis must be limited to avoid side effects. Thus, a lower vector copy number/cell might reduce the risk of genotoxicity. Therefore, different vector doses were tested under rSCF+rTPO and rSCF+rTPO+rFlt3-L stimulation conditions. BaEVTR-LVs and BaEVRLess-LVs transduced significantly higher levels of hCD34+ cells at low vector doses than the other LV pseudotypes (P < .05). They reached 60% (rSCF+rTPO) and 70% (rSCF+rTPO+rFlt3-L) transduction at an MOI of 5, whereas VSV-G-LVs did not even reach 5% transduction (Figure 3B-C). Only at an MOI of 100, the VSV-G-LVs allowed efficient hCD34+ cell transduction rates, which remained far below that of BaEV-LVs (Figure 3B-C). For the strongest cytokine stimulation, BaEVTR- and BaEVRLess-LV transduction reached a plateau at 80% to 90%. Nevertheless, a low vector copy number per cell was revealed (supplemental Table 3). Stable transduction was demonstrated by differentiation of the transduced hCD34+ cells into myeloid lineages (Figure 3D).
Mobilized hCD34+ cells are readily accessible and thus valuable target cells for gene therapy in the clinic. Similar to CB hCD34+ cells, BaEVRless-LVs allowed up to 90% transduction of mobilized hCD34+ cells, and this transduction level persisted in colony forming cells (CFCs) derived from these cells, indicating that short-term progenitors were stably transduced (Figure 3E).
Concluding, BaEV-LVs outperformed VSV-G- and RDTR-LVs by far for hCD34+ cells transduction even when using low cytokine stimulation combined with low vector doses.
BaEV-LVs promote high-level transduction of prestimulated HSCs reconstituting primary and secondary recipient NSG mice
Because of its potential to support high-level engraftment of human cells, we chose the NSG mouse41,42 model to evaluate the long-term reconstitution capacity of BaEV-LV-transduced hCD34+ cells in vivo. To address this question, we transplanted prestimulated CB hCD34+ cells transduced with the different LVs into irradiated NSG mice (Table 1). A comparable high level and variability of engraftment with human cells was detected for all pseudotypes (Table 1; supplemental Figure 4A), indicating that the migration and engraftment of these transduced early progenitors was not impaired.
BaEV-LVs allow high rate transduction of NSG repopulating cells
| Pseudo-type . | BM (%hCD45+) . | BM (%GFP+ cells/total lin+ cells) . | Spleen (% GFP) . | Thymus (% GFP) . | ||||
|---|---|---|---|---|---|---|---|---|
| hCD45 . | hCD34 . | hCD19 . | hCD13 . | hCD14 . | hCD45 . | hCD45 . | ||
| Cytokine-prestimulated hCD34 cells | ||||||||
| VSV-G | ||||||||
| 1 | 22 | 32 | 13 | 34 | 20 | 25 | 24 | 20 |
| 2 | 57 | 6 | 18 | 13 | 22 | 19 | 7 | 2 |
| 3 | 33 | 13 | 9 | 7 | 11 | 15 | 13 | 10 |
| 4 | 43 | 2 | 2 | 0.6 | 0 | 0 | 3 | 0.6 |
| 5 | 74 | 36 | 43 | 30 | 39 | 48 | 26 | 14 |
| RDTR | ||||||||
| 1 | 13 | 37 | 31 | 36 | 41 | 46 | 38 | 27 |
| 2 | 16 | 27 | 33 | 26 | 28 | 25 | 40 | 17 |
| 3 | 26 | 69 | 62 | 78 | 57 | 45 | 38 | 40 |
| 4 | 25 | 12 | 14 | 10 | 11 | 5 | 20 | 16 |
| 5 | 82 | 34 | 41 | 31 | 49 | 40 | 23 | 4 |
| 6 | 25 | 52 | 40 | 56 | 25 | 17 | 52 | 35 |
| BaEVTR | ||||||||
| 1 | 66 | 16 | 19 | 16 | 20 | 16 | 22 | 28 |
| 2 | 53 | 29 | 35 | 24 | 44 | 41 | 38 | 35 |
| 3 | 27 | 92 | 90 | 92 | 82 | 73 | 82 | 83 |
| 4 | 32 | 69 | 57 | 62 | 56 | 71 | 65 | 66 |
| 5 | 46 | 75 | 81 | 72 | 82 | 69 | 69 | 63 |
| 6 | 8 | 88 | 82 | 88 | 80 | 67 | 83 | 66 |
| 7 | 67 | 70 | 73 | 69 | 86 | 80 | 87 | 96 |
| BaEVRless | ||||||||
| 1 | 20 | 40 | 30 | 24 | 44 | 41 | 42 | 42 |
| 2 | 61 | 52 | 56 | 52 | 61 | 60 | 53 | 50 |
| 3 | 89 | 87 | 84 | 87 | 83 | 84 | 70 | 77 |
| 4 | 6 | 95 | 87 | 97 | 75 | 80 | 71 | 84 |
| 5 | 56 | 46 | 54 | 47 | 57 | 47 | 48 | 73 |
| 6 | 72 | 58 | 57 | 58 | 57 | 59 | 53 | 78 |
| 7 | 18 | 72 | 73 | 72 | 69 | 75 | 67 | 70 |
| Unstimulated hCD34 cells | ||||||||
| BaEVTR | ||||||||
| 1 | 71 | 8 | 7 | 6 | 18 | 19 | 19 | 15 |
| 2 | 73 | 8 | 2 | 7 | 6 | 6 | 14 | 1 |
| 3 | 14 | 6 | 8 | 5 | 6 | 6 | 8 | 2 |
| 4 | 81 | 16 | 19 | 16 | 27 | 12 | 19 | 12 |
| 5 | 90 | 14 | 13 | 10 | 15 | 12 | 16 | 8 |
| 6 | 81 | 35 | 27 | 34 | 40 | 42 | 35 | 62 |
| 7 | 61 | 26 | 32 | 35 | 27 | 26 | 18 | 18 |
| BaEVRless | ||||||||
| 1 | 70 | 22 | 20 | 23 | 25 | 20 | 16 | 22 |
| 2 | 75 | 22 | 23 | 27 | 21 | 14 | 26 | 10 |
| 3 | 29 | 11 | 6 | 13 | 11 | 13 | 13 | 58 |
| 4 | 72 | 19 | 19 | 18 | 23 | 19 | 20 | 10 |
| 5 | 64 | 11 | 15 | 12 | 11 | 10 | 23 | 11 |
| 6 | 77 | 36 | 40 | 35 | 39 | 32 | 30 | 54 |
| 7 | 85 | 50 | 55 | 49 | 52 | 56 | 52 | 48 |
| Pseudo-type . | BM (%hCD45+) . | BM (%GFP+ cells/total lin+ cells) . | Spleen (% GFP) . | Thymus (% GFP) . | ||||
|---|---|---|---|---|---|---|---|---|
| hCD45 . | hCD34 . | hCD19 . | hCD13 . | hCD14 . | hCD45 . | hCD45 . | ||
| Cytokine-prestimulated hCD34 cells | ||||||||
| VSV-G | ||||||||
| 1 | 22 | 32 | 13 | 34 | 20 | 25 | 24 | 20 |
| 2 | 57 | 6 | 18 | 13 | 22 | 19 | 7 | 2 |
| 3 | 33 | 13 | 9 | 7 | 11 | 15 | 13 | 10 |
| 4 | 43 | 2 | 2 | 0.6 | 0 | 0 | 3 | 0.6 |
| 5 | 74 | 36 | 43 | 30 | 39 | 48 | 26 | 14 |
| RDTR | ||||||||
| 1 | 13 | 37 | 31 | 36 | 41 | 46 | 38 | 27 |
| 2 | 16 | 27 | 33 | 26 | 28 | 25 | 40 | 17 |
| 3 | 26 | 69 | 62 | 78 | 57 | 45 | 38 | 40 |
| 4 | 25 | 12 | 14 | 10 | 11 | 5 | 20 | 16 |
| 5 | 82 | 34 | 41 | 31 | 49 | 40 | 23 | 4 |
| 6 | 25 | 52 | 40 | 56 | 25 | 17 | 52 | 35 |
| BaEVTR | ||||||||
| 1 | 66 | 16 | 19 | 16 | 20 | 16 | 22 | 28 |
| 2 | 53 | 29 | 35 | 24 | 44 | 41 | 38 | 35 |
| 3 | 27 | 92 | 90 | 92 | 82 | 73 | 82 | 83 |
| 4 | 32 | 69 | 57 | 62 | 56 | 71 | 65 | 66 |
| 5 | 46 | 75 | 81 | 72 | 82 | 69 | 69 | 63 |
| 6 | 8 | 88 | 82 | 88 | 80 | 67 | 83 | 66 |
| 7 | 67 | 70 | 73 | 69 | 86 | 80 | 87 | 96 |
| BaEVRless | ||||||||
| 1 | 20 | 40 | 30 | 24 | 44 | 41 | 42 | 42 |
| 2 | 61 | 52 | 56 | 52 | 61 | 60 | 53 | 50 |
| 3 | 89 | 87 | 84 | 87 | 83 | 84 | 70 | 77 |
| 4 | 6 | 95 | 87 | 97 | 75 | 80 | 71 | 84 |
| 5 | 56 | 46 | 54 | 47 | 57 | 47 | 48 | 73 |
| 6 | 72 | 58 | 57 | 58 | 57 | 59 | 53 | 78 |
| 7 | 18 | 72 | 73 | 72 | 69 | 75 | 67 | 70 |
| Unstimulated hCD34 cells | ||||||||
| BaEVTR | ||||||||
| 1 | 71 | 8 | 7 | 6 | 18 | 19 | 19 | 15 |
| 2 | 73 | 8 | 2 | 7 | 6 | 6 | 14 | 1 |
| 3 | 14 | 6 | 8 | 5 | 6 | 6 | 8 | 2 |
| 4 | 81 | 16 | 19 | 16 | 27 | 12 | 19 | 12 |
| 5 | 90 | 14 | 13 | 10 | 15 | 12 | 16 | 8 |
| 6 | 81 | 35 | 27 | 34 | 40 | 42 | 35 | 62 |
| 7 | 61 | 26 | 32 | 35 | 27 | 26 | 18 | 18 |
| BaEVRless | ||||||||
| 1 | 70 | 22 | 20 | 23 | 25 | 20 | 16 | 22 |
| 2 | 75 | 22 | 23 | 27 | 21 | 14 | 26 | 10 |
| 3 | 29 | 11 | 6 | 13 | 11 | 13 | 13 | 58 |
| 4 | 72 | 19 | 19 | 18 | 23 | 19 | 20 | 10 |
| 5 | 64 | 11 | 15 | 12 | 11 | 10 | 23 | 11 |
| 6 | 77 | 36 | 40 | 35 | 39 | 32 | 30 | 54 |
| 7 | 85 | 50 | 55 | 49 | 52 | 56 | 52 | 48 |
Two- to 3-day-old newborn NOD/SCID γc−/− mice were subjected to a sublethal irradiation of 1 Gy. 2 × 105 hCB-CD34+ cells, transduced at an MOI of 10 with the indicated LV pseudotypes, were injected intrahepatically into the newborns. After 12 weeks of reconstitution, the humanized mice were killed, and the BM, spleen, and thymus were harvested and assessed for levels of GFP+ human cell engraftment (hCD45+GFP+). Multilineage engraftment in the BM was demonstrated by FACS analysis of GFP+ lineage-positive cells. Independent experiments were performed with different hCD34+ cell samples and different batches of each vector.
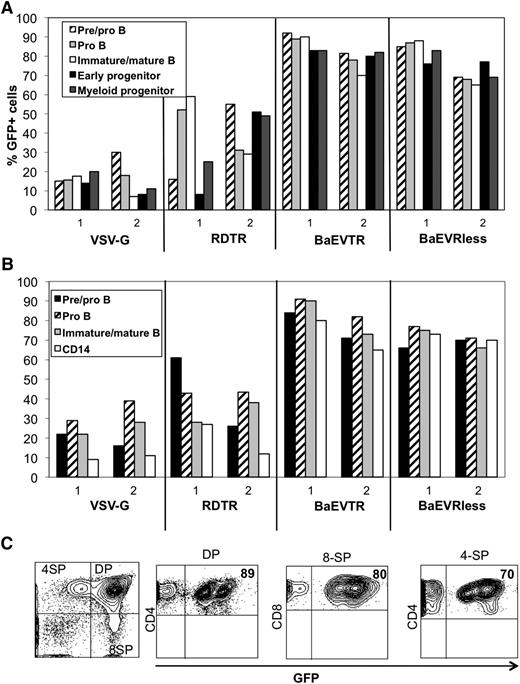
Overall, VSVG-LVs and RDTR-LVs allowed a much lower transduction of SCID repopulating cells (SRCs) compared with BaEV-LVs (Table 1). BaEVTR-LVs- and BaEVRless-LVs-transduced hCD34+ cells resulted in the highest transduction levels of engrafted human cells in the BM (up to 95% CD45+GFP+ cells). These high transduction levels were sustained in the primary recipient mice in all hematopoietic tissues (BM, spleen, and thymus; Table 1). Detailed flow cytometry analysis of the BM revealed equivalent high level transduction for the different B-cell subpopulations (CD19+ pre-B/pro-B, pro-B, and immature/mature B cells), CD13+ myeloid progenitors, and CD14+ monocytes (Figure 4A; supplemental Table 4). For the BaEV-LVs, equivalent high levels of GFP+ early progenitors (CD34+CD19−CD10−) were detected. In contrast, we observed for VSV-G-LVs and RDTR-LVs a high transduction variability between different tissues and the different cell lineages in the BM (Figure 4A; supplemental Table 4). Consistent with these results, detailed phenotyping of the splenocytes revealed a similar transduction profile (Figure 4B). Importantly, the thymocytes (Table 1) and all thymic subpopulations were transduced to the same extent (Figure 4C).
BaEV-LVs efficiently transduce NSG repopulating hCD34+ cells. CB hCD34+ cells were prestimulated for 24 hours with a cytokine-cocktail (TPO+SCF+Flk-3L) and transduced the presence of RetroNectin with RDTR-LVs, BaEVTR-LVs, or BaEVRless-LVs at an MOI of 10 for 36 hours. VSV-G-LV transductions were performed at an MOI = 10. The cells were then injected into the liver of newborn NSG mice. On reconstitution for 12 weeks, the different hematopoietic tissues (BM, spleen, and thymus) of these engrafted mice were analyzed for human cell engraftment by anti-hCD45 staining (Table 1). (A) Transduction levels of the different cell lineages in the BM of NSG reconstituted mice. The percentage of GFP+ immature early progenitor cells (CD34+CD19−CD10−), pre/pro-B cells (CD34+CD19+CD10−), pro-B cells (CD34−CD19+CD10−), immature and mature B cells (CD34−CD19+CD10+), and myeloid progenitors (CD13+) are shown. Data for 2 representative mice from Table 1 for each vector are shown. (B) Transduction levels of different cell lineages in the spleen of NSG reconstituted mice. The percentage of GFP+ pre/pro-B cells (CD34+CD19+CD10−), pro-B cells (CD34−CD19+CD10−), immature and mature B cells (CD34−CD19+CD10+), monocytes, and granulocytes (CD14+) are shown. Data shown correspond to the mice represented in A. (C) Transduction levels of human thymocyte subpopulations of NSG mice reconstituted with hCD34+ cells transduced with BaEVRless-LVs. The percentage of GFP+CD4+CD8+ (DP), CD4+CD8− (4-SP), and CD4−CD8+ (8-SP) is indicated in the upper right quadrant. A representative blot of the BaEVRless mice in Table 1 is shown.
BaEV-LVs efficiently transduce NSG repopulating hCD34+ cells. CB hCD34+ cells were prestimulated for 24 hours with a cytokine-cocktail (TPO+SCF+Flk-3L) and transduced the presence of RetroNectin with RDTR-LVs, BaEVTR-LVs, or BaEVRless-LVs at an MOI of 10 for 36 hours. VSV-G-LV transductions were performed at an MOI = 10. The cells were then injected into the liver of newborn NSG mice. On reconstitution for 12 weeks, the different hematopoietic tissues (BM, spleen, and thymus) of these engrafted mice were analyzed for human cell engraftment by anti-hCD45 staining (Table 1). (A) Transduction levels of the different cell lineages in the BM of NSG reconstituted mice. The percentage of GFP+ immature early progenitor cells (CD34+CD19−CD10−), pre/pro-B cells (CD34+CD19+CD10−), pro-B cells (CD34−CD19+CD10−), immature and mature B cells (CD34−CD19+CD10+), and myeloid progenitors (CD13+) are shown. Data for 2 representative mice from Table 1 for each vector are shown. (B) Transduction levels of different cell lineages in the spleen of NSG reconstituted mice. The percentage of GFP+ pre/pro-B cells (CD34+CD19+CD10−), pro-B cells (CD34−CD19+CD10−), immature and mature B cells (CD34−CD19+CD10+), monocytes, and granulocytes (CD14+) are shown. Data shown correspond to the mice represented in A. (C) Transduction levels of human thymocyte subpopulations of NSG mice reconstituted with hCD34+ cells transduced with BaEVRless-LVs. The percentage of GFP+CD4+CD8+ (DP), CD4+CD8− (4-SP), and CD4−CD8+ (8-SP) is indicated in the upper right quadrant. A representative blot of the BaEVRless mice in Table 1 is shown.
As shown above, BaEVRless-LVs allowed up to 90% transduction of mobilized hCD34+ cells (Figure 3E). These cells allowed high-level engraftment of NSG mice, and the transduction level was sustained in the BM immature progenitors, B cells and myeloid cells, splenocytes, and thymocytes. Detailed phenotyping confirmed that very early progenitor CD34+ cells, as well as B-cell progenitors, myeloid progenitors, and monocytes, in the BM were transduced to the same extent. An identical picture was found for T and B cells and monocytes in the spleen. The different thymic subpopulations showed high-level transduction (supplemental Figure 5).
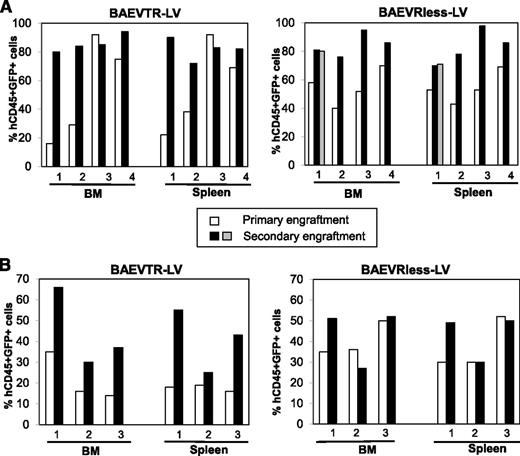
To confirm that true HSCs were gene modified, secondary reconstitutions of NSG mice were performed with hCD34+ cells isolated from primary NSG mice previously reconstituted with BaEVTR- and BaEVRless-LV-transduced prestimulated hCD34+ cells. To improve engraftment into secondary recipients, the hCD34+ cells from the BM of primary NSG mice were cultured, before injection, in SCF and IL-6 to maintain their homing capacity.43 At 12 weeks after transplantation, the BM and spleen of the secondary recipient mice were analyzed for the percentage of hCD45+GFP+ cells (Figure 5A). In the spleen and BM, the percentage of CD45+GFP+ cells was maintained or even increased in the secondary NSG recipients. Importantly, for both BAEVTR- and BAEVRless-LVs, high levels of CD34+GFP+ early progenitors, lymphoid (CD19+), and myeloid (CD13+) cells were detected in the BM of these secondary recipient mice, and equivalent percentages of GFP+ cells were detected in the different lymphoid and myeloid lineages in BM and other hematopoietic tissues (supplemental Figure 6A).
BaEV-LVs transduce efficiently long-term reconstituting HSCs. Transduced cells were injected into the liver of irradiated newborn NSG mice. On reconstitution, the BM and spleen of these primary engrafted mice (first transplantation) were analyzed for transduced human cell engraftment (GFP+CD45+) in the BM and the spleen. Subsequently, the hCD34+ cells, isolated from the BM of these mice, were injected following the same procedure as for the primary NSG engraftments. Ten weeks after engraftment, these secondary recipient mice were analyzed for the percentage of transduced human cells (GFP+CD45+) in the BM and spleen. (A) Analysis of engraftment of transduced human cells (GFP+CD45+) in the BM and spleen of primary transplanted mice reconstituted with prestimulated (TPO+SCF+Flt-3L) hCD34+ cells and transduced with BaEVTR-LVs or BaEVRless-LVs in the presence of RetroNectin and of secondary recipient NSG mice. (B) Analysis of engraftment of transduced human cells (GFP+CD45+) in the BM and spleen of primary and secondary transplanted mice reconstituted with unstimulated hCD34+ cells transduced with BaEVTR-LVs or BaEVRless-LVs in the presence of RetroNectin and of secondary recipient NSG mice.
BaEV-LVs transduce efficiently long-term reconstituting HSCs. Transduced cells were injected into the liver of irradiated newborn NSG mice. On reconstitution, the BM and spleen of these primary engrafted mice (first transplantation) were analyzed for transduced human cell engraftment (GFP+CD45+) in the BM and the spleen. Subsequently, the hCD34+ cells, isolated from the BM of these mice, were injected following the same procedure as for the primary NSG engraftments. Ten weeks after engraftment, these secondary recipient mice were analyzed for the percentage of transduced human cells (GFP+CD45+) in the BM and spleen. (A) Analysis of engraftment of transduced human cells (GFP+CD45+) in the BM and spleen of primary transplanted mice reconstituted with prestimulated (TPO+SCF+Flt-3L) hCD34+ cells and transduced with BaEVTR-LVs or BaEVRless-LVs in the presence of RetroNectin and of secondary recipient NSG mice. (B) Analysis of engraftment of transduced human cells (GFP+CD45+) in the BM and spleen of primary and secondary transplanted mice reconstituted with unstimulated hCD34+ cells transduced with BaEVTR-LVs or BaEVRless-LVs in the presence of RetroNectin and of secondary recipient NSG mice.
Overall, these data strongly suggest that BaEV-LVs allowed genetic modification of very early progenitor cells, hHSCs, allowing long-term engraftment and differentiation into all the different cell lineages in vivo.
BaEVgp-LV pseudotypes permit efficient transduction of resting hCD34+ cells without inducing cell cycle progression
Because BaEVgp-LVs permitted efficient transduction of hCD34+ cells on stimulation with a single cytokine (eg, TPO or SCF; Figure 3A), we hypothesized that their binding and signaling through both aa transporters, ASCT-1 and ASCT-2, might be sufficient to transduce unstimulated hCD34+ cells with the objective to avoid their differentiation and loss of the HSC subpopulation.18,19
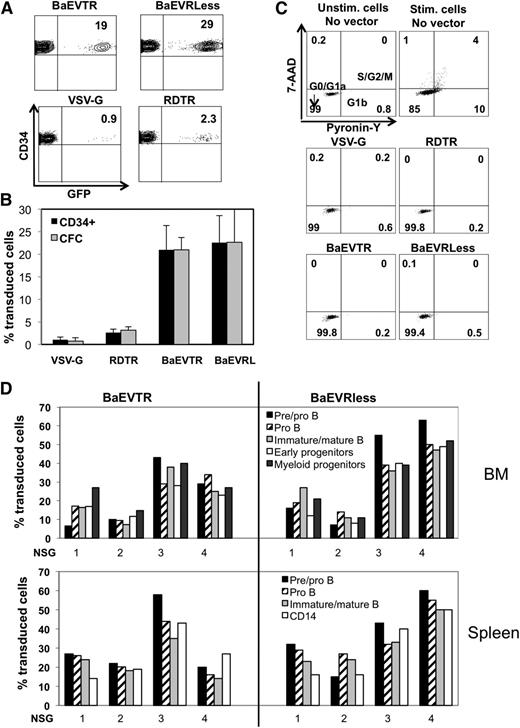
Freshly isolated hCD34+ cells were therefore transduced with BaEVTR-, BaEVRless-, RDTR- and VSV-G-LVs in the absence of exogenous stimuli. Importantly, the BaEVTR- and BaEVRless-LVs were able to efficiently transduce resting hCD34+ cells (15-30%) in the absence of any stimulus following a single exposure at low vector doses (MOI = 10; Figure 6A). In agreement with our previous reports,17,24 the VSV-G-LVs were unable to transduce efficiently resting hCD34+ cells, even when applied at high vector doses (Figure 6A; supplemental Figure 2B). Interestingly, the RDTR-LVs, which bind only to the ASCT-2 receptor, were not able to transduce resting hCD34+ cells efficiently (<5%; Figure 6A). These BaEVgp-LV transduction levels persisted in CFCs derived from the hCD34+ cells, confirming that short-term progenitors were stably transduced (Figure 6B). Important for safety, a low vector copy number per cell was revealed for the BAEV-LVs (supplemental Table 3).
BaEV-LVs are highly superior over RDTR-LVs and VSV-G-LVs for the transduction of unstimulated hCD34+ cells. Freshly isolated CB hCD34+ cells were directly transduced by the indicated LV pseudotypes at an MOI of 10. For VSVG-LVs, an MOI of 100 was used. Analysis for the percentage GFP+ hCD34+-cells was performed 3 days after transduction by FACS. A representative dot blot is shown for each pseudotype in A and the average transduction levels are indicated in B (means ± SD, n = 6). (B) Three days after transduction, the hCD34+ cells were seeded in myeloid differentiation medium. The percentage of GFP+ CFCs is presented (means ± SD, n = 4; P < .001 when comparing BaEV-LVs with VSV-G- and RDTR-LVs). (C) Cell cycle progression was monitored 3 days after transduction of the hCD34+ cells with the different pseudotypes by simultaneously visualizing RNA (Pyronine-Y) and DNA (7-AAD) content of the cells. As controls, unstimulated and stimulated (SCF+TPO+Flt3-L) hCD34+ cells without exposure to vector (no vector) were included. The percentages of cells in the G0/G1a, G1b, and S/G2/M phase of the cell cycle are indicated in the dot blots. (D) Freshly isolated CB hCD34+ cells were transduced with BaEVTR-LVs and BaEVRless-LVs at an MOI = 10 for 24 hours. The cells were then injected into the liver of newborn NSG mice. On reconstitution for 12 weeks, the different hematopoietic tissues (BM, spleen, and thymus) of these engrafted mice were analyzed for human cell engraftment by anti-hCD45 staining (Table 1). Transduction levels of the different cell lineages in the BM of NSG reconstituted mice are presented (upper histograms). GFP+ immature early progenitor cells (CD34+CD19−CD10−), pre/pro-B cells (CD34+CD19+CD10−), pro-B cells (CD34−CD19+CD10−), immature and mature B cells (CD34−CD19+CD10+), and myeloid progenitors (CD13+) are shown. Transduction levels of different cell lineages in the spleen of NSG reconstituted mice (lower histogram). The percentage of GFP+ pre/pro-B cells (CD34+CD19+CD10−), pro-B cells (CD34−CD19+CD10−), immature and mature B cells (CD34−CD19+CD10+), monocytes, and granulocytes (CD14+) are shown. Data for 4 representative mice from Table 1 for each vector type are shown. All transductions were performed in the presence of RetroNectin.
BaEV-LVs are highly superior over RDTR-LVs and VSV-G-LVs for the transduction of unstimulated hCD34+ cells. Freshly isolated CB hCD34+ cells were directly transduced by the indicated LV pseudotypes at an MOI of 10. For VSVG-LVs, an MOI of 100 was used. Analysis for the percentage GFP+ hCD34+-cells was performed 3 days after transduction by FACS. A representative dot blot is shown for each pseudotype in A and the average transduction levels are indicated in B (means ± SD, n = 6). (B) Three days after transduction, the hCD34+ cells were seeded in myeloid differentiation medium. The percentage of GFP+ CFCs is presented (means ± SD, n = 4; P < .001 when comparing BaEV-LVs with VSV-G- and RDTR-LVs). (C) Cell cycle progression was monitored 3 days after transduction of the hCD34+ cells with the different pseudotypes by simultaneously visualizing RNA (Pyronine-Y) and DNA (7-AAD) content of the cells. As controls, unstimulated and stimulated (SCF+TPO+Flt3-L) hCD34+ cells without exposure to vector (no vector) were included. The percentages of cells in the G0/G1a, G1b, and S/G2/M phase of the cell cycle are indicated in the dot blots. (D) Freshly isolated CB hCD34+ cells were transduced with BaEVTR-LVs and BaEVRless-LVs at an MOI = 10 for 24 hours. The cells were then injected into the liver of newborn NSG mice. On reconstitution for 12 weeks, the different hematopoietic tissues (BM, spleen, and thymus) of these engrafted mice were analyzed for human cell engraftment by anti-hCD45 staining (Table 1). Transduction levels of the different cell lineages in the BM of NSG reconstituted mice are presented (upper histograms). GFP+ immature early progenitor cells (CD34+CD19−CD10−), pre/pro-B cells (CD34+CD19+CD10−), pro-B cells (CD34−CD19+CD10−), immature and mature B cells (CD34−CD19+CD10+), and myeloid progenitors (CD13+) are shown. Transduction levels of different cell lineages in the spleen of NSG reconstituted mice (lower histogram). The percentage of GFP+ pre/pro-B cells (CD34+CD19+CD10−), pro-B cells (CD34−CD19+CD10−), immature and mature B cells (CD34−CD19+CD10+), monocytes, and granulocytes (CD14+) are shown. Data for 4 representative mice from Table 1 for each vector type are shown. All transductions were performed in the presence of RetroNectin.
As efficient transduction of hCD34+ cells with classical VSV-G-LVs requires cytokine prestimulation,17 which leads to cell cycle entry (Figure 6C) and probably differentiation, we wanted to exclude that BaEVgp-LV binding to ASCT-1- and -2-induced cell cycle entry. Using a method that allows simultaneous visualization of DNA and RNA,15 we evaluated cell cycle entry on transduction with the different pseudotypes (Figure 6C). As controls, hCD34 -cells were incubated without vector in the absence or presence of cytokine cocktail stimulation. Although BaEVTR- and BaEVRless-LVs resulted in high-level hCD34+ cell transduction, these cells did not actively enter into the cell cycle but resided in the G0/G1a cell cycle phase. This is essential because the majority of the hCD34+ cells with SCID repopulating potential reside in the G0 phase of the cell cycle.44,45
BaEV-LVs promote high-level transduction of resting HSCs
To evaluate whether BaEV-LVs transduced long-term reconstituting HSCs in the resting hCD34+ cell population, we applied a short transduction protocol (18-24 hours) at low vector doses (MOI = 10) without any addition of cytokines before injection into NSG newborn mice. High-level reconstitution with human cells was detected for BaEV-LV-transduced G0 hCD34+ cells, indicating that the homing, engraftment, and differentiation capacity of these cells was not affected (Table 1; supplemental Figure 4). Moreover, the human cell engraftment for BaEV-LV-transduced G0 hCD34+ cells was significantly higher than for their prestimulated counterparts (supplemental Figure 4; P < .01). Interestingly, for the low (8-20%) and high (>30%) transduction efficiencies, equivalent transduction rates in all the different lineages in the BM and spleen were revealed (Table 1; Figure 6D; supplemental Table 5).
Importantly, both ASCT-1 and ASCT-2 mRNA levels are up-regulated on cytokine stimulation (supplemental Figure 7A-B), which might strongly increase the affinity of BaEV-LVs to hCD34+ cells and explain higher transduction levels in these stimulated cells compared with their resting counterparts.
Secondary reconstitutions of NSG mice were performed as for the prestimulated hCD34+ cells. The percentage of CD45+GFP+ cells in the BM and spleen was maintained or even increased by 1.5- to threefold in the secondary recipient mice (Figure 5B). Importantly, for both BaEV-LVs, high levels of transduced CD34+ early progenitors and lymphoid (CD19+) and myeloid (CD13+) cells were detected in the BM of these secondary recipient mice, and equivalent percentages of GFP+ cells were detected in the other hematopoietic tissues (supplemental Figure 6B).
In summary, these BaEVgp-LVs allowed genetic modification of very early progenitor repopulating cells, so-called hHSCs in the unstimulated hCD34+ cell population, able to home, engraft, and differentiate into all the different lineages in primary and secondary NSG mice.
Discussion
We describe for the first time efficient pseudotyping of LVs with the envelope glycoprotein BaEV, derived from the baboon endogenous virus. Like RDTR-LV pseudotypes,25 they are stable in human and macaque sera, which offers potential clinical perspectives for in vivo gene therapy. These BaEV-LVs did not only allow up to 90% transduction of prestimulated hCD34+ SRCs at low vector doses but also high transduction levels of quiescent hCD34+ SRCs, relevant for clinical application, whereas RDTR- or VSV-G-LVs performed very poorly in this latter condition. Moreover, in using BaEV-LVs, we would be able to significantly decrease the vector doses without a significant loss of gene transfer efficiency, which could make this treatment accessible to a higher number of patients.
During engraftment, transplanted HSCs home to the BM niche, where they initiate both differentiation and self-renewal. There, they reside in a hypoxic niche, where they acquire dormancy and maintain stemness.42,46 It might be, therefore, crucial to not overexpose the hCD34+ HSCs to cytokines, currently required for high-level transduction with VSV-G-LVs18,19 Indeed, recent clinical trials7-10 still use strong cytokine cocktails, although this can affect the cell cycle status and the integrity of the HSC pool.47-49 Importantly, compared with hCD34+ cells in G0, cells in G1 or SG2M phases of the cell cycle demonstrated diminished engraftment.44,48,50,51 Here, we show that BaEV-LVs achieve high transduction rates (60-90%) of hCD34+ cells when they are prestimulated briefly with rSCF, rTPO, or a combination of rSCF and rTPO. These mild cytokine stimulation protocols might strongly reduce the risk of HSC differentiation, cell cycle entry, and homing impairment and better conserve HSC function. In agreement, we previously confirmed that LVs displaying only the survival cytokine SCF at their surface transduced long-term SRCs.22-24 Remarkably, our results indicated that BaEVgp-LVs can transduce up to 30% of unstimulated hCD34+ cells, whereas VSV-G-LVs and RDTR-LVs remained inefficient. Thus, the former vectors might improve HSC transduction and conserve HSC potential, a combination needed for successful treatment of diseases in which gene-corrected repopulating cells have no selective advantage (eg, hemoglobinopathies). Although a higher level of HSC correction and conservation is of benefit for all monogenetic diseases, patients with Fanconi anemia would in particular benefit from resting hCD34+ cell transduction. Indeed, the Fanconi anemia genetic defect causes fragility of the hCD34+ cells, which are strongly reduced in number in these patients.52 Moreover, cytokine stimulation induces Fanconi anemia hCD34+ cells into apoptosis. Therefore, minimizing hCD34+ cell stimulation combined with efficient gene transfer would be invaluable for Fanconi anemia gene therapy.11,53,54
That the transduction performance of BaEV-LVs is highly superior to the ones obtained by VSV-G-LVs, especially in quiescent hCD34+ cells, is not surprising because we recently solved this mystery by demonstrating that unstimulated hCD34+ cells do not express the VSV receptor LDL-R.16 Even after a short-term cytokine stimulation, only a subpopulation up-regulated the LDL-R, limiting VSV-G-LV transduction.17 The receptor for RD114, ASCT-2,31,32,55 is up-regulated in cytokine-prestimulated hCD34+ cells compared with unstimulated cells56 (supplemental Figure 7), which might explain the low RDTR-LV transduction levels in quiescent hCD34+ cells, although some ASCT-2 expression is detected.57,58 The RDTR-LV transduction levels obtained for cytokine-stimulated hCD34+ cells reached a plateau at 50% in agreement with many other reports.25,56,59-62 Remarkably, the BaEV-LVs allowed hCD34+ cell transduction levels to significantly increase (80-90%) cytokine stimulation compared with RDTR-LVs, and they even achieved efficient transduction of these cells without any stimulation. Most probably, this increase must be attributed to the fact that, in contrast to RD114, BaEV recognizes the ASCT-1 receptor in addition to ASCT-2 on the hCD34+ cell population.34 Indeed, both receptor mRNA levels are up-regulated cytokine stimulation (supplemental Figure 7), which might increase the affinity of BaEV-LV binding to hCD34+ cells.
Currently, LVs for gene therapy are produced by transient transfection. To date, one important instrument missing is a stable LV packaging cell line. This proved more difficult than expected because some viral proteins are toxic for 293T-HEK producer cells such as the VSV-G envelope gp.63 Several stable cell lines were engineered; however, they still are not used in the clinic.64-68 Importantly, 2 stable cell lines for high-titer RDTR-LV production were engineered.30,69 This points toward BaEVTR-gp as an important candidate for establishing a stable LV packaging cell line because its long-term expression is not cytotoxic to 293T cells (Figure 1D), and these vectors allow high-level HSC transduction. Of note, we confirmed that these BaEV-LVs also allow high-level gene transfer in human T and B cells (A.G-G. and E.V., unpublished data, June 2013).
Summarizing, the new BaEV-LVs allowed improvement of HSC gene transfer levels, accompanied by conservation of HSC function. They transduce unstimulated hCD34+ cells with NSG long-term repopulating capacity at high levels that are relevant for gene therapy. Therefore, these new pseudotypes can improve current clinical protocols for HSC-based gene therapy to assure lifelong cure.
The online version of this article contains a data supplement.
There is an Inside Blood Commentary on this article in this issue.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
The authors thank the staff of the animal care facility (Plateau de Biologie Expérimentale de la Souris) at the École normale supérieure (ENSa) de Lyon, the flow cytometry and vectorology platform (UMS3444/US8, Lyon, France). The authors also thank Ornélie Bernadin for help with experiments.
This work was supported by the following grants: Association Française contre les Myopathies, Agence Nationale de Recherche sur le Sida, Agence de Recherche sur le Cancer, and the European Community (FP7-HEALTH-2007-B/222878 “PERSIST” and E-RARE-06-01 “GETHERTHAL”).
Authorship
Contribution: E.V. coordinated the project, designed and performed experiments, analyzed and discussed the data, and wrote the manuscript; A.G.-G. and F.A. designed and performed experiments, analyzed the data, discussed results; C.L., C.F., C.C., F.F., and D.N. performed experiments; D.L. provided reagents, critical discussion, and technical advice; and F.-L.C. provided critical discussion and reading of the manuscript.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Els Verhoeyen, CIRI/EVIR, ENS de Lyon, 46 Allée d’Italie, 69364 Lyon Cedex 07, France; e-mail: els.verhoeyen@ens-lyon.fr; or François-Loïc Cosset, CIRI/EVIR, ENS de Lyon, 46 Allée d’Italie, 69364 Lyon Cedex 07, France; e-mail: flcosset@ens-lyon.fr.
References
Author notes
A.G.-G. and F.A. contributed equally to this work.

![Figure 1. Modification of the cytoplasmic tails of BaEV glycoproteins allows efficient pseudotyping of lentiviral vectors. (A) Schematic representation of the WT (BaEVwt) and mutant BaEV (BaEVTR, BaEVRless), RDTR- and MLV-A gps. The 17 aa long cytoplasmic domain of the cat and baboon retroviral gps, RD114 and BaEVwt, respectively, was exchanged for one of the MLV-A glycoproteins resulting in the chimeric RDTR- and BaEVTR-gps, respectively. The R peptide of the cytoplasmic tail of BaEVwt was deleted, resulting in the BaEVRLess mutant gps. Amino acid sequences and different domains for these gps are shown in supplemental Figure 1. (B) Titers of the different pseudotyped LVs encoding GFP, obtained by infection of HEK293T cells with serial dilutions of fresh or 100-fold concentrated vector preparations (infectious units/mL). The percentage of GFP+ cells was determined 3 days after infection by FACS (means ± standard deviation [SD], n = 8; ***P < .005). (C) Immunoblots of LV particles displaying the different BaEV-gps at their surface. LVs were purified over a sucrose cushion by ultracentrifugation. The upper part shows staining with antibodies against the surface domain of BaEVwt. The corresponding HIV-1 capsid was revealed to verify equal loading. The positions of the BaEVgps and the HIV-1 capsid (HIV1-CA) are indicated. (D) Pictures show 293T cells 48 hours after transfection with BaEVTR- and BaEVRless-gps.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/124/8/10.1182_blood-2014-02-558163/4/m_1221f1.jpeg?Expires=1767713020&Signature=przmfJPaaih2PUkxL9TVLhXczIgYevrs8Gv86AFbsY3i4YqzRWaVwotqjcaHE8Gh1YW2aj8oQcpNWVAtTamw8arqIxzYqlYvdOUrWy6gRSYUIim2hT4M4n0A57t1XX6sC5x05Lpd4gpyFwSRVwANSDM7K6b~9IARhZwCmO9g-ZIWiC30I~IH5pmhF3tzxQNICzTQpBK2WJSdHIpmuAdjOWPYISokFcs~lSNrBN~zGyPYBB3Ar6zqGpib2TWKffpQ6lXyT7YY6oDA4DB23FRWOFlEDEpjrXg4AKZkC7ntVqoyyDcafwx71dQspfqO15lUzsgmUwXTC4ftf0B57sJxcA__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)