Key Points
CRP enhances IgG-mediated respiratory burst and phagocytosis of platelets in vitro and their clearance in vivo.
CRP levels are increased in ITP patients and correlate with platelet counts and bleeding severity and predict time to recovery.
Abstract
Immune-mediated platelet destruction is most frequently caused by allo- or autoantibodies via Fcγ receptor-dependent phagocytosis. Disease severity can be predicted neither by antibody isotype nor by titer, indicating that other factors play a role. Here we show that the acute phase protein C-reactive protein (CRP), a ligand for Fc receptors on phagocytes, enhances antibody-mediated platelet destruction by human phagocytes in vitro and in vivo in mice. Without antiplatelet antibodies, CRP was found to be inert toward platelets, but it bound to phosphorylcholine exposed after oxidation triggered by antiplatelet antibodies, thereby enhancing platelet phagocytosis. CRP levels were significantly elevated in patients with allo- and autoantibody-mediated thrombocytopenias compared with healthy controls. Within a week, intravenous immunoglobulin treatment in children with newly diagnosed immune thrombocytopenia led to significant decrease of CRP levels, increased platelet numbers, and clinically decreased bleeding severity. Furthermore, the higher the level of CRP at diagnosis, the longer it took before stable platelet counts were reached. These data suggest that CRP amplifies antibody-mediated platelet destruction and may in part explain the aggravation of thrombocytopenia on infections. Hence, targeting CRP could offer new therapeutic opportunities for these patients.
Introduction
Fetal or neonatal alloimmune thrombocytopenia (FNAIT) and immune thrombocytopenia (ITP) are both antibody-mediated disorders in which platelets are destroyed mainly through activating immunoglobulin (IgG) Fc receptors on phagocytes in the spleen and liver, eventually resulting in thrombocytopenia.1 In childhood, ITP is characterized by a typical history of acute development of purpura and bruising in an otherwise healthy child, caused by development of platelet autoantibodies, with an incidence of ∼5 in 100 000 children.2 Most children with newly diagnosed ITP will not suffer from serious bleeding and will recover within 12 months. In ∼60% of ITP, there is a history of a prior infection.1-3 FNAIT is a potentially destructive disease in pregnancies, with intracerebral hemorrhage (ICH) of the fetus or neonate as the most feared complication, resulting in perinatal death in 1% to 7% or in severe neurological impairments in 14% to 26% of affected pregnancies.4-7 FNAIT is caused by maternal IgG platelet alloantibodies, in whites mainly directed against human platelet antigen (HPA)-1a (85%), that cross the placenta and destroy the platelets of the fetus or newborn. Immunization against HPA-1a occurs in ∼1:450 random pregnancies.8-10 Although platelet decrement is related to antibody titer in FNAIT,8,11,12 this correlation is not strict, as cases with low titers and very low platelet counts, as well as cases with high titers and normal platelet counts, are frequently observed. Recently we found that at least some of this discrepancy is due to differences in the functional quality of these antibodies, determined by its Fc glycosylation, in particular the level of core fucosylation.13 However, the data indicated that additional cofactors may also be involved.
Here we identified C-reactive protein (CRP), a member of the pentraxin family of highly conserved calcium-dependent ligand-binding proteins, to amplify IgG-mediated platelet destruction. CRP is composed of 5 identical, nonglycosylated 206 amino acids protomers forming a noncovalently linked annular symmetrical pentamer.14 CRP was originally described by Tillett et al in 1930 as a substance present in the sera of patients with acute inflammation, precipitating with cell wall (C) polysaccharide (CWPS) of Streptococcus pneumonia.15 CRP is now well established as a major acute phase protein and is used in daily clinical practice as a sensitive biomarker for infection and inflammation, with its level increasing from <0.05 to >500 mg/L after acute infections. CRP is produced by hepatocytes, in response to inflammatory cytokines such as interleukin (IL)-6 and IL-1, with serum concentrations rising to >5 mg/L after 6 hours and peaking after ∼48 hours. In healthy young adult volunteer blood donors, the median concentration of CRP was found to be ∼0.8 mg/L14 and does not seem to cross the placenta.16,17
In the present paper, we describe how CRP interacts directly with platelets and functions as a novel pathogenic cofactor in IgG-mediated platelet destruction by phagocytes, ultimately leading to platelet destruction in patients that can, at least partially, be overcome by treatments aiming at lowering CRP levels.
Methods
Clinical trial of intravenous immunoglobulin (IVIg) treatment in newly diagnosed ITP in children
Details are described in the supplemental Methods, available on the Blood Web site, and patient characteristics are summarized in supplemental Table 1.
Induction of thrombocytopenia in vivo
Six-week-old female BALB/c mice (strain BALB/cOlaHsd) were obtained from Harlan and were housed in our animal facility for 1 week before initiating experiments. Thrombocytopenia was induced in mice, as we described previously.18 Briefly, 0.75 µg rat anti-mouse CD41, clone MWReg30, was injected intraperitoneally with or without various concentrations of CRP. Other details are mentioned in the supplemental Methods.
Sera, reagents, cell isolations, labeling, and heparin-induced platelet activation test
Due to length restrictions, these sections are described in the supplemental Methods.
Phagocytosis and respiratory burst assays
Phagocytosis and respiratory burst experiments were carried out using freshly isolated human platelets and polymorphonuclear leukocytes (PMN) or sorted CD16+ monocytes. For phagocytosis, platelets were labeled with pHrodo as described in the supplemental Methods, a pH-sensitive fluorescent dye only emitting a fluorescent signal at acid pH.13 Equal volumes of 2.0 × 106/mL PMN and 1.0 × 108/mL platelets were mixed in a total volume of 100 µL in 1.4-mL U-bottom tubes (Micronic, Lelystad, The Netherlands), for 20 minutes in a shaking incubator at 37°C. Respiratory burst experiments were carried out in exactly the same way only using unlabeled platelets and with dihydrorhodamine 123 (Invitrogen, Molecular Probes, Eugene, OR) added to PMN before mixing with platelets, at a final concentration of 1 µM. In addition, the incubation at 37°C was carried out for 45 minutes unless otherwise indicated. When platelets were used without preopsonization, they were incubated directly in the indicated media or 1:10 diluted sera. After the reaction, the cells were kept on ice and washed in cold phosphate-buffered saline (PBS). Samples were measured in a flow cytometer (LSRII; BD Bioscience, San Jose, CA) and analyzed by FacsDIVA software (BD Bioscience).
Surface plasmon resonance
Surface plasmon resonance (SPR) measurements analyzing protein-protein interactions were carried out in an IBIS MX96 (IBIS Technologies, Enschede, The Netherlands) as described by de Lau et al.19 Cellular SPR (cSPR) measuring the interaction of whole platelets with biosensor-coupled protein ligands was carried out on the IBIS MX96 using methods based on the principles described in Schasfoort et al.20 Both are described in details in the supplemental Methods.
Statistical analysis
Statistical analysis was performed using GraphPad Prism 5.01 software for Windows (GraphPad Software, San Diego, CA) with a statistical significance at P < .05.
Results
A factor in normal human serum is required for phagocyte responses toward IgG-opsonized platelets
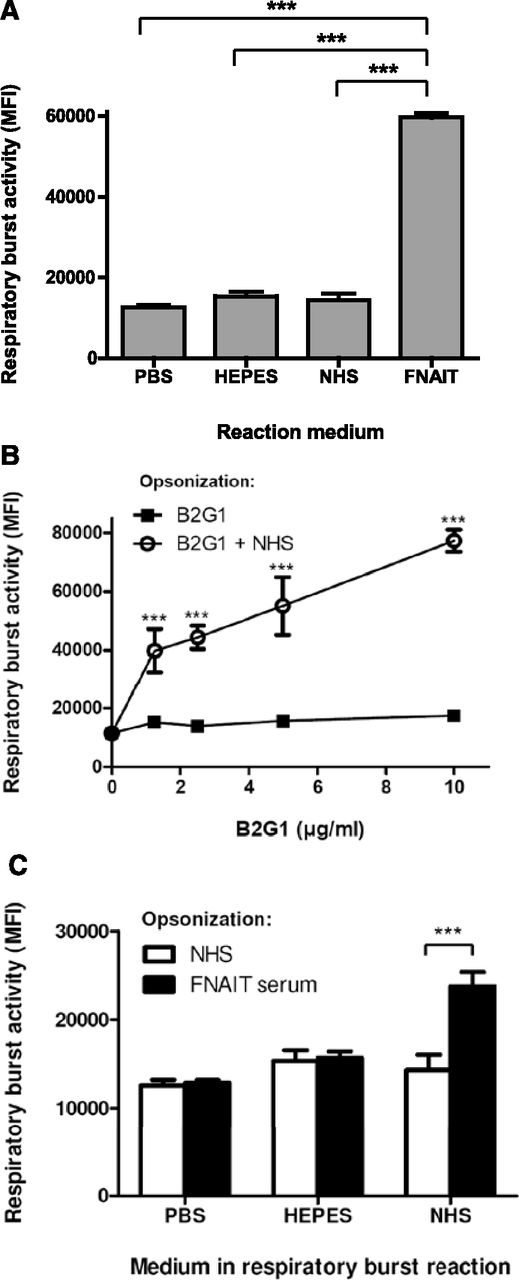
In vitro handling of platelets (eg, labeling for phagocytosis experiments) can lead to platelet activation, resulting in background signals from phagocytosis of nonopsonized platelets (supplemental Figure 1). By measuring respiratory burst (nicotinamide adenine dinucleotide phosphate, reduced NAD phosphate [NADPH], oxidase activity) of neutrophils by flow cytometry, platelet handling was minimized, and therefore background signals originating from normal human serum (NHS) or buffered saline solutions were eliminated, whereas platelets opsonized with anti-HPA-1a IgG1-containing FNAIT serum displayed a strong phagocyte response (Figure 1A). We then tested whether the phagocyte responses observed with HPA-1a–specific FNAIT sera could be simulated with a human recombinant HPA-1a–specific monoclonal IgG1 antibody (B2G1).21 No respiratory burst was observed, unless NHS was added during the assay (Figure 1B). The same was true for platelets washed after opsonization with maternal FNAIT sera containing anti-HPA-1a antibodies, as no antibody-dependent respiratory burst was observed, unless NHS was added after washing (Figure 1C). This suggests a factor to be present in NHS that is required for antibody-mediated phagocyte responses toward platelets.
Normal human sera contain a factor required for phagocyte responses toward IgG-opsonized platelets. (A) Respiratory burst activity of PMN toward platelets in NHS or anti-HPA-1a antibody-containing FNAIT sera or saline solutions, analyzed by the conversion of dihydrorhodamine-1,2,3 to fluorescent rhodamine-1,2,3, measured by fluorescence-activated cell sorter. (B-C) PMN respiratory burst activity toward IgG-opsonized platelets was only observed in NHS. (B) Platelets were opsonized with the human monoclonal anti-HPA-1a IgG1 antibody; B2G1, subsequently washed, was resuspended in either PBS, HEPES, or NHS together with PMN to initiate phagocyte responses. (C) Platelets were preincubated with either NHS (unopsonized) or opsonized with FNAIT serum, washed with PBS, and resuspended in the indicated media before addition to PMN, and then the respiratory burst was measured after 45 minutes at 37°C. Data are representative of 3 independent experiments, showing mean ± standard deviation. Statistical comparisons were performed as follows: (A) 1-way analysis of variance (ANOVA) with Tukey’s posttest; (B-C) 2-way ANOVA with Bonferroni posttest. ***P ≤ .001.
Normal human sera contain a factor required for phagocyte responses toward IgG-opsonized platelets. (A) Respiratory burst activity of PMN toward platelets in NHS or anti-HPA-1a antibody-containing FNAIT sera or saline solutions, analyzed by the conversion of dihydrorhodamine-1,2,3 to fluorescent rhodamine-1,2,3, measured by fluorescence-activated cell sorter. (B-C) PMN respiratory burst activity toward IgG-opsonized platelets was only observed in NHS. (B) Platelets were opsonized with the human monoclonal anti-HPA-1a IgG1 antibody; B2G1, subsequently washed, was resuspended in either PBS, HEPES, or NHS together with PMN to initiate phagocyte responses. (C) Platelets were preincubated with either NHS (unopsonized) or opsonized with FNAIT serum, washed with PBS, and resuspended in the indicated media before addition to PMN, and then the respiratory burst was measured after 45 minutes at 37°C. Data are representative of 3 independent experiments, showing mean ± standard deviation. Statistical comparisons were performed as follows: (A) 1-way analysis of variance (ANOVA) with Tukey’s posttest; (B-C) 2-way ANOVA with Bonferroni posttest. ***P ≤ .001.
Serum factor is heat resistant, found at variable levels in NHS, and requires Ca2+ for platelet binding
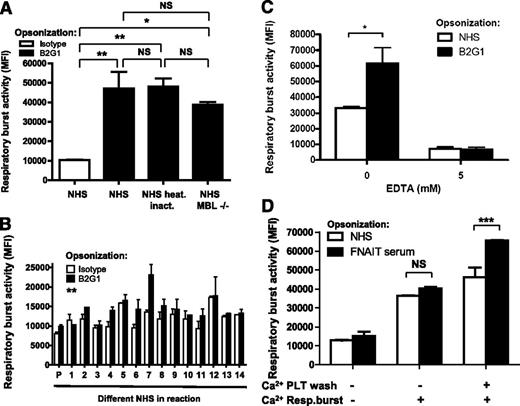
Serum heat inactivation at 56°C for 30 minutes had no significant effect on the respiratory burst (Figure 2A; supplemental Figure 2), ruling out the requirement of complement. Furthermore, B2G1-opsonized platelets still demonstrated the serum-enhancing effect in sera deficient for mannan-binding lectin (Figure 2A). When testing 14 different healthy donors, we observed a variable degree of serum-dependent respiratory burst activity toward B2G1-opsonized platelets (Figure 2B), also evident in a time-dependent fashion (supplemental Figure 3), suggesting the responsible serum component to be present at different levels in NHS. Next, we tested whether this factor required divalent cations for its function. Addition of EDTA to NHS completely abolished the serum-enhancing effect on the B2G1-mediated respiratory burst (Figure 2C). No IgG-specific respiratory burst was observed when the opsonizing FNAIT serum was washed away without Ca2+, even if Ca2+ was readded after washing during the respiratory burst. An IgG-specific antiplatelet response was only observed if Ca2+ was present during the washing step (Figure 2D), indicating that the responsible serum factor reacts directly and reversibly with anti-HPA-1a–opsonized platelets in a calcium-dependent manner.
Enhancing effect of serum is not complement, varies between NHS, and requires divalent cations for interaction with platelets. (A) Respiratory burst activity of PMN toward B2G1-opsonized platelets in NHS was not diminished after heat inactivation of complement. NHS, deficient in mannan-binding lectin, was also capable of enhancing phagocyte responses to B2G1-opsonized platelets. NHS served as a negative control. (B) The respiratory burst activity of PMN toward B2G1-opsonized platelets was enhanced by some but not all 14 different NHS compared with unopsonized platelets. (C) Chelation of divalent cations from NHS with EDTA (5 mM) ablates the ability of serum to induce respiratory burst of PMN triggered by B2G1-opsonized platelets. (D) The serum factor, enhancing IgG-specific PMN respiratory burst activity toward platelets binds directly to platelets in a calcium-dependent manner. Platelets were preincubated with NHS or anti-HPA-1a FNAIT sera, washed with HEPES with or without Ca2+, and resuspended in HEPES with or without Ca2+ as indicated. Data are representative of 3 independent experiments, showing mean ± standard deviation. Statistical comparisons were performed as follows: (A) 1-way ANOVA with Tukey’s posttest; (B) paired t test; and (C-D) 2-way ANOVA with Bonferroni posttest. *P ≤ .05; **P ≤ .01; ***P ≤ .001.
Enhancing effect of serum is not complement, varies between NHS, and requires divalent cations for interaction with platelets. (A) Respiratory burst activity of PMN toward B2G1-opsonized platelets in NHS was not diminished after heat inactivation of complement. NHS, deficient in mannan-binding lectin, was also capable of enhancing phagocyte responses to B2G1-opsonized platelets. NHS served as a negative control. (B) The respiratory burst activity of PMN toward B2G1-opsonized platelets was enhanced by some but not all 14 different NHS compared with unopsonized platelets. (C) Chelation of divalent cations from NHS with EDTA (5 mM) ablates the ability of serum to induce respiratory burst of PMN triggered by B2G1-opsonized platelets. (D) The serum factor, enhancing IgG-specific PMN respiratory burst activity toward platelets binds directly to platelets in a calcium-dependent manner. Platelets were preincubated with NHS or anti-HPA-1a FNAIT sera, washed with HEPES with or without Ca2+, and resuspended in HEPES with or without Ca2+ as indicated. Data are representative of 3 independent experiments, showing mean ± standard deviation. Statistical comparisons were performed as follows: (A) 1-way ANOVA with Tukey’s posttest; (B) paired t test; and (C-D) 2-way ANOVA with Bonferroni posttest. *P ≤ .05; **P ≤ .01; ***P ≤ .001.
CRP is the unknown serum factor
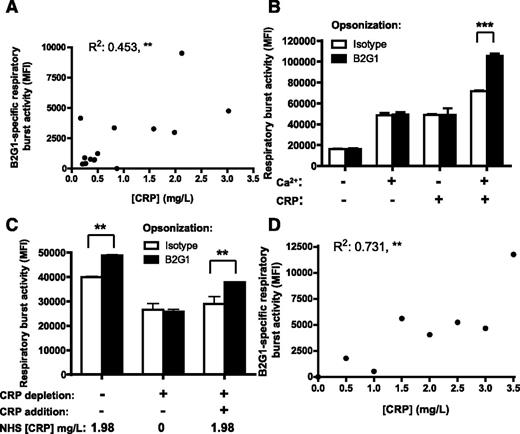
Due to the variable magnitude of the response observed in different NHS and the calcium dependency, we hypothesized that CRP could be this unknown factor. Further analysis of the previously tested 14 NHS indeed demonstrated a significant correlation between the IgG-specific respiratory burst activity and CRP concentration of the NHS (Figure 3A). We then tested whether addition of CRP enabled phagocyte responses toward B2G1-opsonized platelets. We only observed respiratory burst toward IgG-opsonized platelets in the presence of both CRP and calcium; either component alone was not sufficient (Figure 3B). Depletion of CRP from NHS with phosphorylcholine beads (Figure 3C) also resulted in loss of the B2G1-induced respiratory burst activity. After readding purified CRP to the CRP-depleted NHS, the B2G1-specific effect was restored (Figure 3C). Also, addition of CRP to NHS with very low CRP levels (0.07 mg/L) resulted in a significant increase of the respiratory burst in a concentration-dependent manner (Figure 3D), similar to seen in different NHS (Figure 3A). These data indicate that CRP in the serum was responsible for enhancing phagocyte responses toward IgG-opsonized platelets.
CRP enhances phagocyte responses of PMN toward IgG-opsonized platelets. (A) B2G1-specific respiratory burst activity of 14 NHS correlates significantly with the CRP concentrations in the sera. (B) Both CRP and calcium were required to induce IgG-specific respiratory burst. B2G1-opsonized platelets were incubated in HEPES supplemented with or without calcium and in the presence or absence of CRP, before addition of PMN to measure the respratory burst. (C) Depletion from NHS with phosphocholine-coated beads neutralized its ability to enhance IgG1-mediated respiratory burst toward B2G1-opsonized platelets, but resupplementing CRP restored this capacity. (D) CRP addition to NHS with low CRP (0.07 mg/L) rendered the serum capable of inducing IgG-specific respiratory burst toward B2G1-opsonized platelets that correlated significantly with the amount of CRP added. Data are representative of 3 independent experiments, showing mean ± standard deviation. Statistical comparisons were performed as follows: (A,D) Pearson correlation; (B-C) 2-way ANOVA with Bonferroni posttest. *P ≤ .05; **P ≤ .01; ***P ≤ .001.
CRP enhances phagocyte responses of PMN toward IgG-opsonized platelets. (A) B2G1-specific respiratory burst activity of 14 NHS correlates significantly with the CRP concentrations in the sera. (B) Both CRP and calcium were required to induce IgG-specific respiratory burst. B2G1-opsonized platelets were incubated in HEPES supplemented with or without calcium and in the presence or absence of CRP, before addition of PMN to measure the respratory burst. (C) Depletion from NHS with phosphocholine-coated beads neutralized its ability to enhance IgG1-mediated respiratory burst toward B2G1-opsonized platelets, but resupplementing CRP restored this capacity. (D) CRP addition to NHS with low CRP (0.07 mg/L) rendered the serum capable of inducing IgG-specific respiratory burst toward B2G1-opsonized platelets that correlated significantly with the amount of CRP added. Data are representative of 3 independent experiments, showing mean ± standard deviation. Statistical comparisons were performed as follows: (A,D) Pearson correlation; (B-C) 2-way ANOVA with Bonferroni posttest. *P ≤ .05; **P ≤ .01; ***P ≤ .001.
Fc-dependent platelet activation exposes CRP ligands, recognized by CRP phosphorylcholine-binding sites
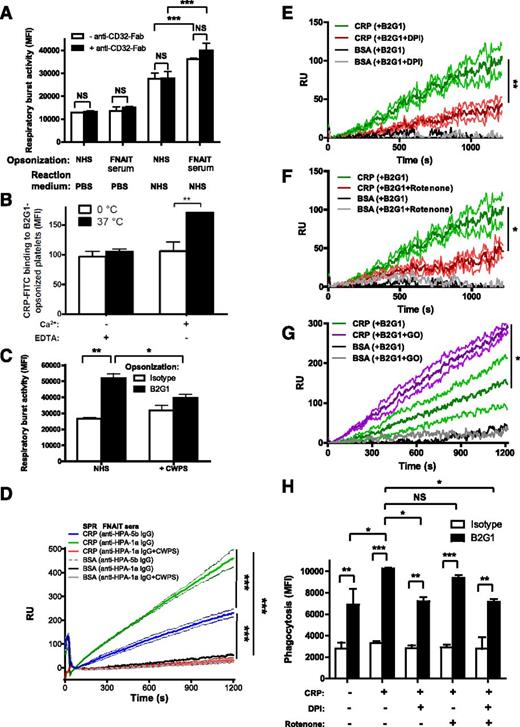
We then investigated whether the binding of anti-HPA-1a antibody led to platelet activation and exposure of CRP ligands after co-crosslinking with platelet FcγRIIa (the Kurlander phenomenon).22 However, blocking anti-FcγRII (clone 7.3)-Fab had no effect on the respiratory burst induced by platelets opsonized with FNAIT-derived IgG (Figure 4A) or with B2G1 (supplemental Figure 4). FcγRIIa-blocking capacity of 7.3-Fab was verified in the FcγRIIa-dependent heparin-induced platelet activation assay23,24 (supplemental Figure 5). We next sought to determine whether CRP bound directly to IgG-opsonized platelets as indicated by the results presented in Figure 2D. It has been suggested that CRP can directly bind to C1q,25 which we confirm by SPR, where C1q bound CRP captured by anti-CRP antibody (supplemental Figure 6A-B). However, CRP binding to C1q is unlikely to explain the mechanism of CRP enhancement of phagocyte responses toward IgG1-opsonized platelets, as the effect was observed with purified CRP in the absence of serum (Figure 3B-E) and after serum heat inactivation, for which C1q is sensitive26 (Figure 2A). In further support of this, we did not find C1q binding to spotted IgG1 to facilitate additional CRP binding (supplemental Figure 6B-C), nor did captured C1q by spotted anti-C1q antibodies provide a platform for CRP binding (supplemental Figure 6B-C). Furthermore, CRP also did not bind IgG1 directly (supplemental Figure 6A). When fluorescein isothiocyanate-labeled CRP was incubated with B2G1-opsonized platelets, weak but significant binding of CRP was observed at 37°C in the presence of calcium, but not at 0°C or in the absence of calcium (Figure 4B). This confirmed that the calcium-dependent binding domains of CRP were involved in the binding to the platelets, but also that platelet activation was required to initiate binding. To determine the binding site of CRP on platelets, NHS was preincubated with pneumococcal CWPS, which binds to the phosphorylcholine-ligand binding site on CRP in calcium-dependent manner.27 This resulted in a reduced IgG-specific respiratory burst (Figure 4C). To investigate the binding of CRP to platelets more closely, we used a sensitive cSPR method that we recently developed for red blood cells but now applied to platelets.20 With this method, the platelets were allowed to interact with up to 48 spots, each coupled with different ligands, and the platelet interaction to each spot was measured simultaneously in real time. Platelets (HPA-1a+/HPA-5b−) opsonized with anti-HPA-1a FNAIT sera bound to CRP-conjugated spots, but not to control bovine serum albumin-conjugated spots, to a significantly greater extent than nonopsonized platelets (incubated with anti-HPA-5b FNAIT sera; Figure 4D). The interaction of platelets with spotted CRP was completely blocked with CWPS (Figure 4D), which bound to the CRP spots (supplemental Figure 7). A similar binding to CRP and blockage by CWPS was also demonstrated using platelets opsonized with B2G1 (supplemental Figure 8). However, CWPS was unable to break the binding of platelets already bound to CRP coupled to SPR chips (data not shown), probably due to the nature of high avidity interactions. To test the Fc dependency of our mechanism, we conducted cSPR experiments comparing CRP binding of platelets opsonized with either intact B2G1 or B2G1-F(ab′)2. The binding of platelets to CRP appeared to be Fc dependent, as platelets opsonized with B2G1-F(ab′)2 fragments bound significantly less to CRP than platelets opsonized with the full-length B2G1 (supplemental Figure 9). Taken together, the data suggest that CRP binds membrane phosphorylcholine residues on IgG-opsonized platelets exposed after platelet activation induced by anti-HPA-1a–specific antibodies.
Cellular activation exposes phoshorylcholine after oxidation, enabling CRP binding and phagocytosis. (A) Platelet FcγRIIa was not involved in the opsonization of platelets with FNAIT serum. Platelets were pretreated with blocking anti-CD32-Fab antibody (clone 7.3), opsonized with anti-HPA-1a–containing FNAIT sera (NHS as negative control), washed, and resuspended in PBS (negative control) or NHS. Thereafter, the platelets were added to PMN and the respiratory burst was measured. (B) CRP fluorescein isothiocyanate bound directly to B2G1-opsonized platelets, and this process required both calcium and platelet activity at 37°C, as measured by flow cytometry. (C) Neutralization of CRP with pneumococcal CWPS in NHS disabled CRP induction of the respiratory burst toward B2G1-opsonized platelets. (D) Binding of FNAIT serum-opsonized platelets (HPA-1a+/HPA-5b−) to CRP was further investigated by cSPR imaging, with CRP spotted to 3 sensor surface spots and 3 bovine serum albumin control spots. A specific response was observed for platelets to the CRP spots, which was enhanced if the platelets were opsonized with anti-HPA-1a antibodies from FNAIT serum (also compared with platelets incubated with anti-HPA-5b antibodies from FNAIT serum), but blocked by CWPS, indicating that platelet phosphorylcholine is the ligand for CRP that is exposed by antibody binding. (E) Binding of platelets to CRP was inhibited by DPI, a broad spectrum oxidation inhibitor including NADPH oxidase, as well as by Rotenone, which more specifically inhibits the mitochondrial electron transport chain (F). Conversely, stimulation of platelet oxidation by glucose and glucose oxidase enhanced platelet binding to CRP (G). Each line in D-G represents the average sensorgrams and standard deviation from 3 spots monitored simultaneously in real time. Statistical comparisons were performed as follows: (H) CRP enhancement of IgG-mediated phagocytosis of platelets by neutrophils was inhibited by DPI and not by Rotenone. Data are representative of 3 independent experiments, showing mean ± standard deviation. (A,C,D,H) 1-way ANOVA with Tukey’s posttest; (B) 2-way ANOVA with Bonferroni posttest; (E-G) 1-tailed paired t test. *P ≤ .05; **P ≤ .01; ***P ≤ .001.
Cellular activation exposes phoshorylcholine after oxidation, enabling CRP binding and phagocytosis. (A) Platelet FcγRIIa was not involved in the opsonization of platelets with FNAIT serum. Platelets were pretreated with blocking anti-CD32-Fab antibody (clone 7.3), opsonized with anti-HPA-1a–containing FNAIT sera (NHS as negative control), washed, and resuspended in PBS (negative control) or NHS. Thereafter, the platelets were added to PMN and the respiratory burst was measured. (B) CRP fluorescein isothiocyanate bound directly to B2G1-opsonized platelets, and this process required both calcium and platelet activity at 37°C, as measured by flow cytometry. (C) Neutralization of CRP with pneumococcal CWPS in NHS disabled CRP induction of the respiratory burst toward B2G1-opsonized platelets. (D) Binding of FNAIT serum-opsonized platelets (HPA-1a+/HPA-5b−) to CRP was further investigated by cSPR imaging, with CRP spotted to 3 sensor surface spots and 3 bovine serum albumin control spots. A specific response was observed for platelets to the CRP spots, which was enhanced if the platelets were opsonized with anti-HPA-1a antibodies from FNAIT serum (also compared with platelets incubated with anti-HPA-5b antibodies from FNAIT serum), but blocked by CWPS, indicating that platelet phosphorylcholine is the ligand for CRP that is exposed by antibody binding. (E) Binding of platelets to CRP was inhibited by DPI, a broad spectrum oxidation inhibitor including NADPH oxidase, as well as by Rotenone, which more specifically inhibits the mitochondrial electron transport chain (F). Conversely, stimulation of platelet oxidation by glucose and glucose oxidase enhanced platelet binding to CRP (G). Each line in D-G represents the average sensorgrams and standard deviation from 3 spots monitored simultaneously in real time. Statistical comparisons were performed as follows: (H) CRP enhancement of IgG-mediated phagocytosis of platelets by neutrophils was inhibited by DPI and not by Rotenone. Data are representative of 3 independent experiments, showing mean ± standard deviation. (A,C,D,H) 1-way ANOVA with Tukey’s posttest; (B) 2-way ANOVA with Bonferroni posttest; (E-G) 1-tailed paired t test. *P ≤ .05; **P ≤ .01; ***P ≤ .001.
Platelet oxidation exposes CRP ligands
As oxidized low-density lipoprotein (LDL) particles and apoptotic cells have been shown to expose phosphatidylcholine residues,28,29 and because anti-GPIIIa (containing the HPA-1a epitope, the target of the FNAIT sera and of the B2G1 antibody used in this study) antibodies have been shown to induce oxidation of platelets,30 we investigated whether platelet oxidation contributed to CRP binding. Pretreatment of platelets with diphenylene iodonium (DPI), an inhibitor of various sources of oxygen radical including NADPH oxidase,31 resulted in less binding of opsonized platelets to CRP compared with untreated opsonized platelets (Figure 4E). A similar reduction in binding of platelets to CRP was observed in the presence with Rotenone, a compound more specifically inhibiting the mitochondrial electron transport chain (Figure 4F). Conversely, stimulation of oxidation of opsonized platelets after treatment with glucose oxidase (Figure 4G) resulted in enhanced binding of opsonized platelets to CRP spots compared with untreated opsonized platelets.
As CRP also enhanced antibody-mediated phagocytosis, measuring complete ingestion of B2G1-opsonized platelets (Figure 4H), we were able to investigate the role of oxidation in antibody-mediated platelet ingestion. In agreement with the finding that oxidation was required for binding of CRP to platelets, CRP-mediated enhancement of phagocytosis was completely inhibited by DPI treatment, but not after Rotenone treatment, suggesting the phagocyte NADPH oxidase to be mostly responsible for binding of CRP to platelets during phagocytosis (Figure 4H). Although CRP clearly enhanced phagocytosis, we also noticed that B2G1 was able to mediate phagocytosis without CRP (Figure 4G), unlike for the respiratory burst where CRP seemed to be indispensable for the IgG-specific response (Figure 3B), underlining the different thresholds and different cellular mechanisms required for activation of these different processes.
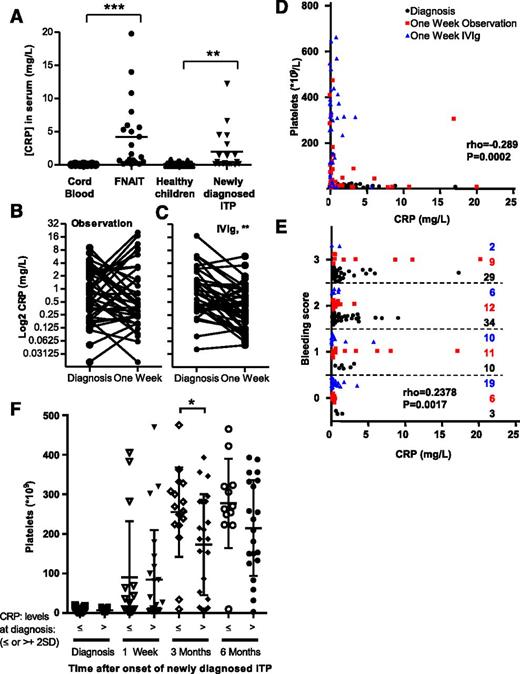
CRP is elevated in ITP, is associated with platelet counts and clinical bleeding severity, and predicts time to recovery
To examine the relevance of CRP in patient sera, we measured the CRP concentration in neonatal HPA-1a-FNAIT patient samples and in sera from children with newly diagnosed ITP (all with confirmed anti-GPIIb/IIIa antibodies) and compared this to normal cord blood or sera of healthy children, respectively. CRP levels were increased in both FNAIT and newly diagnosed ITP patient populations compared with control samples (Figure 5A). To further investigate whether the level of CRP affects the bleeding tendency in these patients, we made use of the ongoing Treatment With or Without IVIg in Kids With Acute ITP trial. In this trial, patients with newly diagnosed immune thrombocytopenia were randomized on diagnosis to the observation arm or to those receiving 0.8 g/kg IVIg and followed in time. At inclusion, all patients had <20 × 109 platelets/L. One week after receiving IVIg, most patients had normalized their number of platelets, whereas this was not the case for the observation group (supplemental Figure 10). The elevated CRP levels seen in these patients (Figure 5A) also became significantly lower in the IVIg group but not in the control group (Figure 5B-C). Both the number of platelets and their bleeding tendencies correlated significantly with the CRP levels in these patients (Figure 5D-E, respectively). For both groups as a whole, those individuals who had normalized their number of platelets to the international consensus level (>100 × 109 /L)32 after 1 week from diagnosis also had significantly lower CRP levels compared with those with thrombocytopenia (<100 × 109/L; supplemental Figure 11). In untreated patients, those with elevated CRP levels at diagnosis (greater than mean + 2 × standard deviation of healthy control children; Figure 5A) were more likely to have sustained lowered platelet counts 3 months after diagnosis (Figure 5F). This is in line with our hypothesis that CRP is an important amplifier of antibody-mediated platelet destruction in immune thrombocytopenic patients.
CRP levels are elevated in immune thrombocytopenic patients and decreased by IVIg treatment, resulting in normalization of platelet counts and reduction of clinical bleeding severity. (A) In humans, CRP levels were also elevated in neonatal FNAIT sera compared with healthy cord blood samples (both n = 21) and sera from newly diagnosed ITP patients compared with age-matched healthy controls (both n = 19). (B-E) Seventy-eight newly diagnosed ITP pediatric patients, all with <20 × 109 platelets/L, were randomized for observation or to receive 0.8 g/kg IVIg. (B) CRP levels did not change significantly in the observation group. (C) However, IVIg treatment caused significant reduction in CRP levels. (D) IVIg treatment also resulted in increased numbers of platelets, which correlated significantly with CRP levels. (E) Similarly, CRP levels correlated significantly with bleeding tendencies, ranging from 0 to 5 (0, no bleeding; 5, life threatening) according to Buchanan et al.68 The symbol key for D-E is as follows: black circles, patients when enrolled in the study (diagnosis); red squares, untreated arm 1 week later (1-week observation); blue triangles, IVIg-treated arm 1 week later (1-week IVIg). (F) Elevated levels of CRP at diagnosis predict slower platelet count recovery. Untreated newly diagnosed ITP pediatric patients were retrospectively categorized into normal CRP levels or elevated CRP levels (defined as higher than mean CRP levels of healthy children + 2 times their standard deviation). Statistical comparison was performed with (A) 2-tailed Mann-Whitney test, (B) 2-tailed paired Wilcoxon, (D-E) 1-tailed Spearman’s rank correlation, and (F) 2-tailed Student t test after testing for Gaussian distribution. *P ≤ .05; **P ≤ .01; ***P ≤ .001.
CRP levels are elevated in immune thrombocytopenic patients and decreased by IVIg treatment, resulting in normalization of platelet counts and reduction of clinical bleeding severity. (A) In humans, CRP levels were also elevated in neonatal FNAIT sera compared with healthy cord blood samples (both n = 21) and sera from newly diagnosed ITP patients compared with age-matched healthy controls (both n = 19). (B-E) Seventy-eight newly diagnosed ITP pediatric patients, all with <20 × 109 platelets/L, were randomized for observation or to receive 0.8 g/kg IVIg. (B) CRP levels did not change significantly in the observation group. (C) However, IVIg treatment caused significant reduction in CRP levels. (D) IVIg treatment also resulted in increased numbers of platelets, which correlated significantly with CRP levels. (E) Similarly, CRP levels correlated significantly with bleeding tendencies, ranging from 0 to 5 (0, no bleeding; 5, life threatening) according to Buchanan et al.68 The symbol key for D-E is as follows: black circles, patients when enrolled in the study (diagnosis); red squares, untreated arm 1 week later (1-week observation); blue triangles, IVIg-treated arm 1 week later (1-week IVIg). (F) Elevated levels of CRP at diagnosis predict slower platelet count recovery. Untreated newly diagnosed ITP pediatric patients were retrospectively categorized into normal CRP levels or elevated CRP levels (defined as higher than mean CRP levels of healthy children + 2 times their standard deviation). Statistical comparison was performed with (A) 2-tailed Mann-Whitney test, (B) 2-tailed paired Wilcoxon, (D-E) 1-tailed Spearman’s rank correlation, and (F) 2-tailed Student t test after testing for Gaussian distribution. *P ≤ .05; **P ≤ .01; ***P ≤ .001.
CRP enhances antiplatelet IgG-mediated destruction of platelets in vivo
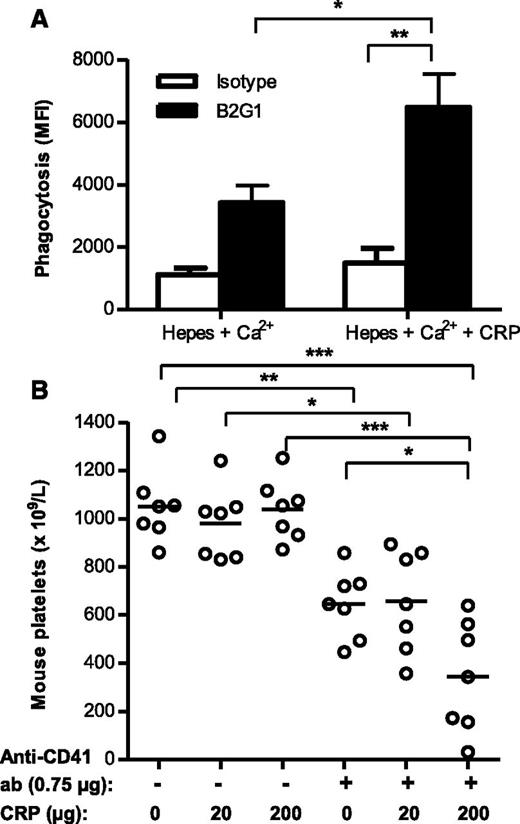
We next investigated whether the elevated CRP levels directly contribute to platelet degradation in vivo. As monocytes and macrophages have been generally proposed to be more relevant effector cells for clearance of the platelets in the spleen, we first tested whether CRP also enhances monocyte-mediated phagocytosis of platelets. In line with the results described above, monitoring the respiratory burst and phagocytosis by PMN, we found CRP also to stimulate phagocytosis of B2G1-opsonized platelets by monocytes (Figure 6A). We next examined whether the observed effects of CRP in antibody-mediated platelet destruction also occurred in vivo, using our previously established mouse model for immune-mediated thrombocytopenia.18 Coadministration of 200 μg CRP, together with a limiting dose of 0.75 µg of antiplatelet MWReg30 IgG (GPIIb), significantly decreased the mean platelet counts compared with injection of 0.75 µg of antiplatelet IgG alone, whereas administration of 200 μg CRP alone had no effect on platelet counts (Figure 6B). Coadministration of a lower amount of CRP (20 μg) together with antiplatelet IgG did not result in a significant decrease in platelet counts. Taken together, we conclude that CRP can potentiate the degree of thrombocytopenia induced by antiplatelet antibodies in vivo.
CRP enhances antibody-mediated platelet destruction in vitro and in vivo. (A) Phagocytosis of pHrodo-labeled platelets opsonized with either isotype control or B2G1 antibody for 20 minutes at 37°C for CD16+ monocytes. (B) BALB/c mice developed thrombocytopenia 16 hours after intraperitoneal injection of the platelet- and megakaryocyte-specific rat anti-mouse CD41 IgG at 0.75 µg. Coinjection of 200 µg CRP with 0.75 µg rat anti-mouse CD41 IgG resulted in aggravated thrombocytopenia after 16 hours, whereas 200 µg CRP alone had no effect. Data are representative of 3 independent experiments; each data symbol represents 1 mouse (7 per group). Statistical comparisons were performed by 1-way ANOVA with Tukey’s posttest. *P ≤ .05; **P ≤ .01; ***P ≤ .001.
CRP enhances antibody-mediated platelet destruction in vitro and in vivo. (A) Phagocytosis of pHrodo-labeled platelets opsonized with either isotype control or B2G1 antibody for 20 minutes at 37°C for CD16+ monocytes. (B) BALB/c mice developed thrombocytopenia 16 hours after intraperitoneal injection of the platelet- and megakaryocyte-specific rat anti-mouse CD41 IgG at 0.75 µg. Coinjection of 200 µg CRP with 0.75 µg rat anti-mouse CD41 IgG resulted in aggravated thrombocytopenia after 16 hours, whereas 200 µg CRP alone had no effect. Data are representative of 3 independent experiments; each data symbol represents 1 mouse (7 per group). Statistical comparisons were performed by 1-way ANOVA with Tukey’s posttest. *P ≤ .05; **P ≤ .01; ***P ≤ .001.
Discussion
In this study, we describe a previously unrecognized role for CRP in IgG-mediated cellular destruction. Similar to that of other studies, eg, in the proposed role of CRP in enhancing the risk of cardiovascular disease,33 the results here suggest a significant role for CRP in patients with elevated basal levels of CRP.
We observed that anti-HPA-1a antibodies induce exposure of CRP ligands on platelets due to oxidative damage. This effect was not dependent on the platelet FcγRIIa, but required an intact Fc, perhaps suggesting that antibodies mediate platelet activation through Fc-mediated GPIIb/IIIa crosslinking by IgG hexamerization as recently shown to occur on IgG-opsonized particles and cells.34 That anti-HPA-1a antibodies induce platelet oxidation is in agreement with studies showing that anti-GPIIIa antibodies, the glycoprotein expressing the HPA-1a epitope, also induce cellular activation through oxidative damage initiated by the platelet NADPH oxidase.30,35,36 This suggests this effect to be a general feature of antibodies targeting GPIIIa—perhaps the GPIIb/IIIa complex—as we now also report CRP also amplify platelet removal by rat-anti-mouse GPIIb. Importantly, this still needs to be investigated in detail using a panel of antiplatelet antibodies against various GPIIb/IIIa epitopes, ITP sera containing various combinations of antibodies, and against other platelet proteins. However, our findings suggesting CRP levels to be an alternative prognostic marker for thrombocytopenia are in line with studies demonstrating a role for oxidative stress in ITP,37,38 as well as studies describing oxidation-dependent lipid changes of LDL and apoptotic cells to expose phosphatidylcholines to the extracellular milieu for recognition by CRP.28,39 Choline-containing CWPS blocked binding of CRP to platelets and prevented CRP-mediated enhancement of antiplatelet antibody-mediated respiratory burst and phagocytosis. Moreover, inhibiting oxidation reduced binding of CRP to IgG-opsonized platelets. Conversely, stimulation of platelet oxidation increased binding to CRP. Inhibiting the mitochondrial respiratory chain with Rotenone was sufficient to block this initial oxidation on the platelet surface and subsequent CRP deposition. However, only DPI (a broad spectrum blocker of oxidation, including NADPH oxidase) was capable of blocking CRP-mediated phagocytosis, whereas rotanone had no effect. It is therefore likely that the more powerful NADPH oxidase system of the phagocyte is primarily responsible for providing additional oxidized CRP ligands, further enhancing platelet phagocytosis.
Infections, viral or bacterial, are known to promote the initiation of ITP or enhance platelet clearance, but the exact underlying mechanisms are unknown. Previously, one of the possible mechanisms suggested is the molecular mimicry between platelet antigens and various viral and bacterial antigens, giving rise to cross-reactive autoantibodies.36,40-42 In addition, 14 of 23 (61%) of the patients with Helicobacter pylori-associated ITP demonstrated an increased platelet count after the H pylori had been eradicated.43 As H pylori is a gram-negative bacterium, these findings are also in line with the effects of lipopolysaccharide (LPS), a gram-negative bacterial endotoxin, which was shown to enhance FcγR-mediated phagocytosis of IgG-opsonized platelets in vitro44 and to exert a strong synergistic effect in vivo.45 LPS may therefore be 1 factor explaining why the thrombocytopenia worsens in ITP patients during gram-negative infections, but also why symptoms are alleviated after the infection is resolved. Although the mechanisms of this LPS effect remains unknown, it has been suggested that LPS triggers either phagocyte and/or platelet Toll-like receptor 4, inducing synergistic signaling through FcγR and thereby enhanced platelet clearance.46,47
The work presented here provides an alternative explanation, suggesting endotoxin exposure to increase CRP levels through the acute phase responses. The endotoxin itself, low levels of antiplatelet antibodies, or the combination may lead to platelet activation, followed by oxidation and subsequent phosphorylcholine exposure, providing a binding platform for CRP. Low-level IgG opsonization of platelets, not sufficient to initiate phagocytosis on its own, can then be enhanced by CRP, providing a ligand for additional phagocyte FcγR, but also FcαRI (the IgA receptor), as both receptor types have been shown to bind CRP,48-54 further marking it for phagocyte destruction. These observations are also in line with recent reports indicating that oxidative stress correlates with prognosis in ITP patients,37,38 perhaps as a result of continuing release of oxidative products during phagocyte-mediated clearance of IgG/CRP-opsonized platelets, promoting further platelet clearance by excessive CRP deposition onto platelets.
A number of studies have reported bleeding events to be associated with increased levels of CRP, eg, in acute coronary syndromes55 and ICH.56 CRP levels were even found to predict early hematoma outgrowth after ICH.57 In addition, peptic ulcers have been reported to upregulate IL-6,58 with a subsequent rise is CRP levels, which in turn may predict gastrointestinal bleeding events.59-61 Additionally, we previously found that anti-HPA-1a antibodies can reduce endothelial cell spreading and monolayer integrity, possibly affecting bleeding tendency.62 As CRP has also been shown to have a direct proinflammatory effect on endothelial cells,63 CRP may boost the anti-HPA-1a–mediated response amplifying bleeding. These studies did not confirm whether CRP is the cause or consequence for these bleeding events. In our FNAIT cohort, we found no indications that elevated CRP levels are causing severe bleeding events, as ICH only occurs in ∼7% to 26% of the cases,64 whereas CRP levels were found to be elevated in ≥50% of our randomly selected cases. We also observed cases with high levels of CRP who did not display clinical symptoms and vice versa. ICH also occurred in cases without elevated CRP. However, in our newly diagnosed ITP group as a whole, the presence of elevated CRP did correlate with more severe clinical manifestations, and the higher the CRP levels were at diagnosis, the longer it took to reach stabile platelet counts. The increased CRP levels may also possibly sustain the elevated CRP levels through a proinflammatory feedback loop as previously suggested,65 thereby also explaining the slower recovery. This could perhaps also be used to decide which patients warrant treatment of their ITP. More direct evidence for the causality between increased CRP levels and enhanced platelet degradation was obtained in mice, where CRP alone was inert against platelets but enhanced thrombocytopenia together with antiplatelet antibodies, also in line with results obtained from phagocytosis of platelets.
We found that widespread treatment used in ITP, IVIg, which has been proposed to exert its action through numerous ways,66 was also associated with a reduction in the CRP levels, and those levels significantly correlated with both the number of platelets and clinical bleeding severity. IVIg is also able to protect mice from LPS-exacerbated antibody-mediated thrombocytopenia, albeit to a lower degree than without LPS.45
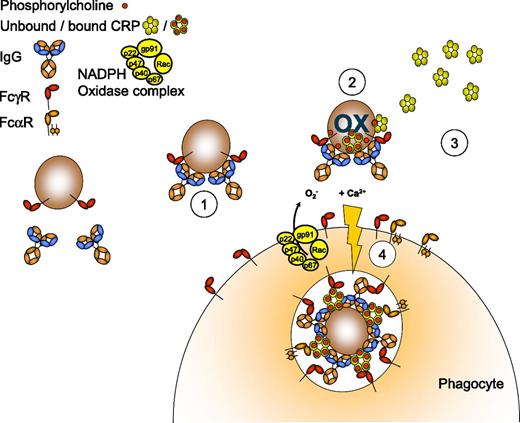
In summary, we conclude that CRP functions as a previously unknown pathogenic cofactor that contributes to antibody-mediated platelet destruction (a model is provided in Figure 7). Increased CRP levels at diagnosis predicted slower platelet count recovery in ITP, and the CRP levels dropped after IVIg treatment, accompanied by normalization of platelet counts and decreased clinical bleeding severity. As such, these data also suggested a possible mechanism for the exacerbation or relapse of ITP associated with infections, as infections may be associated with an increase in CRP, indicating that CRP may serve as an important biomarker for monitoring severities of IgG-mediated thrombocytopenias. This suggests that treatment of the underlying cause of the inflammation in addition to treatment of the thrombocytopenia itself would be beneficial for the patient. Importantly, FNAIT and/or ITP patients may benefit from interventions aimed at reducing CRP levels or inhibiting its function.67
Illustration of the proposed role for CRP in IgG-mediated platelet destruction. Antiplatelet IgG antibodies from serum bind to platelets (1). Platelet oxidation (OX) results in phosphorylcholine exposure, independently of platelet FcγRIIa, but requires the phagocyte NADPH oxidation system (2). Subsequently, CRP present in the serum binds to platelet phosphorylcholine in a Ca2+-dependent manner (3). This potentiates the uptake and degradation of the platelets via Fc receptors of the phagocyte (4).
Illustration of the proposed role for CRP in IgG-mediated platelet destruction. Antiplatelet IgG antibodies from serum bind to platelets (1). Platelet oxidation (OX) results in phosphorylcholine exposure, independently of platelet FcγRIIa, but requires the phagocyte NADPH oxidation system (2). Subsequently, CRP present in the serum binds to platelet phosphorylcholine in a Ca2+-dependent manner (3). This potentiates the uptake and degradation of the platelets via Fc receptors of the phagocyte (4).
The online version of this article contains a data supplement.
There is an Inside Blood Commentary on this article in this issue.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
The authors thank Prof Dr René van Lier for critically reading the manuscript, Cedric Ghevaert and Prof Dr Willem Ouwehand for the generous gift of the purified B2G1 antibody, and Anton Tool for the supply of the oxidation inhibitor DPI used in the study. R.K. was supported by Sanquin Product and process development for cellular products grant-09-025.
Authorship
Contribution: R.K. performed the experiments, some together with A.E.H.B. and R.V.; M.C.A.B. and K.M.J.H.-P. supervised the clinical trial; R.K. and G.V. wrote the manuscript; G.V. initiated and supervised the study; and all authors conceived of or aided with experiments, analyzed data, edited the manuscript, and prepared figures.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Gestur Vidarsson, Department of Experimental Immunohematology, Sanquin Research and Landsteiner Laboratory, Plesmanlaan 125, 1066 CX Amsterdam, The Netherlands; e-mail: g.vidarsson@sanquin.nl.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal