In this issue of Blood, de Groot et al identify a hydrophobic pocket in the Cys-rich domain of ADAMTS13 that appears to interact with the hydrophobic pocket in the central A2 domain of von Willebrand factor (VWF) for its proteolysis.1
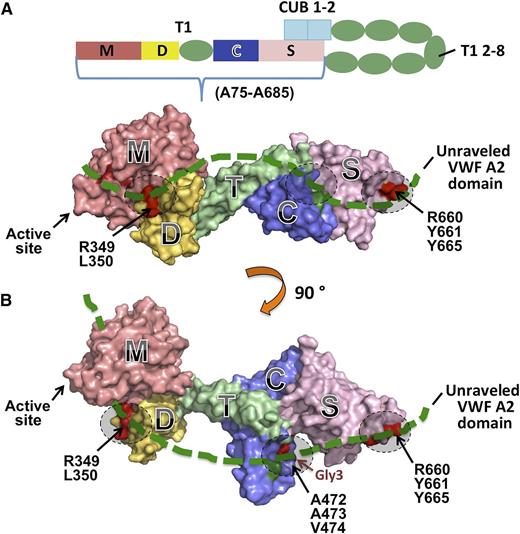
(A) Schematic representation of human ADAMTS13 domain structure. The C-terminal TSP1 (T1) 2-8 and CUB domains are proposed to interact with the spacer domain. (B) Two different views of the 3-dimensional structure of the MDTCS fragment. Here, M, the metalloprotease domain, is modeled; the disintegrin domain (D), first thrombospondin type 1 repeat (T), Cys-rich domain (C), and spacer domain (S) are crystallized.11 A part of the unraveled central A2 domain of VWF (thick green dotted line) may interact with MDTCS on 3 identified exosites (dashed circles) including the surface charged residues R660, Y661, and Y665 in the spacer domain, a hydrophobic pocket involving A472, A473, V474, and H476 (H476N/Q478T, Gly3) in the Cys-rich domain, and a nonconserved region involving R349 and L350 in the disintegrin domain. Adapted from Figure 6 in the article by de Groot et al that begins on page 1968.
(A) Schematic representation of human ADAMTS13 domain structure. The C-terminal TSP1 (T1) 2-8 and CUB domains are proposed to interact with the spacer domain. (B) Two different views of the 3-dimensional structure of the MDTCS fragment. Here, M, the metalloprotease domain, is modeled; the disintegrin domain (D), first thrombospondin type 1 repeat (T), Cys-rich domain (C), and spacer domain (S) are crystallized.11 A part of the unraveled central A2 domain of VWF (thick green dotted line) may interact with MDTCS on 3 identified exosites (dashed circles) including the surface charged residues R660, Y661, and Y665 in the spacer domain, a hydrophobic pocket involving A472, A473, V474, and H476 (H476N/Q478T, Gly3) in the Cys-rich domain, and a nonconserved region involving R349 and L350 in the disintegrin domain. Adapted from Figure 6 in the article by de Groot et al that begins on page 1968.
ADAMTS13, a circulating blood metalloenzyme, cleaves an unusually large adhesion protein, VWF, which is released from endothelium after injury. This proteolytic cleavage is essential for maintaining a delicate balance between normal hemostatic function and abnormal platelet agglutination (or thrombosis). Severe deficiency of plasma ADAMTS13 activity, resulting from ADAMTS13 mutations or acquired autoantibodies that inactivate ADAMTS13, leads to a potentially fatal syndrome: thrombotic thrombocytopenic purpura (TTP).2 Mild to moderate deficiency of plasma ADAMTS13 activity or increased ratios of VWF to ADAMTS13 have been shown to be risk factors for the development of systemic inflammation, myocardial or cerebral infarction, preeclampsia or eclampsia, and cerebral malaria.3
In the last decade, significant progress has been made toward the understanding of the structural and functional relationships of ADAMTS13 and VWF. The data available to date suggest that the recognition and productive cleavage of VWF depend on the amino-terminal portion of ADAMTS13 (ie, metalloprotease, disintegrin, first thrombospondin type 1 repeat, cysteine-rich and spacer [MDTCS] domains; residues Ala75-Ala685; see figure panel A). The role of more distal domains of ADAMTS13 from the second to the eighth thrombospondin type 1 repeat plus 2 CUB domains (T2C) is still not fully understood. We and others have shown that T2C may be dispensable or required for binding to native/soluble VWF and endothelium-bound ultra large VWF.4 Interestingly, more recent studies by kinetic analyses indicate that T2C, particularly the CUB domains, may play a regulatory role by inhibition of ADAMTS13 activity through their potential interaction with the spacer domain (see panel A). This was shown by an approximate twofold increase in proteolytic activity after T2C or 2 CUB domains were deleted or after addition of a monoclonal antibody that bound to the CUB domains.5,6 Shear-induced unfolding of the VWF A2 domain or acidic pH appears to mitigate the inhibition by the carboxyl-terminal tail.5,6 In vivo, there is no apparent difference in antiarterial thrombotic activity between full-length ADAMTS13 and the truncated MDTCS variant for inhibition of the formation of ultra-large VWF strings and the rate of thrombus formation in murine models of mesenteric arterial thrombosis.7 How the CUB domains interact with the spacer or other domains to mediate their inhibitory activity remains an open question.
It is now well accepted that the metalloprotease domain alone is not sufficient to cleave VWF and its peptide analogs. Addition of disintegrin, the first thrombospondin type 1 repeat, Cys-rich, and spacer domains sequentially to the metalloprotease domain progressively increases its proteolytic activity,8 suggesting that each of these amino-terminal domains is critical for substrate recognition. Binding experiments have demonstrated that each individual amino-terminal domain (except the metalloprotease domain) appears to bind VWF73 with appreciable affinities (KD, ∼100-500 µM), but the MDTCS domains together bind VWF73 with much higher affinity (KD, ∼7 nM).8 Furthermore, a large8 or small9 deletion or even a point mutation9,10 in any of these noncatalytic domains results in significant impairment of ADAMTS13 activity. Together, these findings suggest that the MDTCS domains work in concert for substrate recognition and proteolysis.
de Groot et al elegantly demonstrate a hydrophobic pocket in the Cys-rich domain of ADAMTS13 that appears to directly interact with a hydrophobic pocket in the central A2 domain as being 2 complementary binding sites critical for ADAMTS13 and VWF interaction. First, by modification of several potential glycan attaching sites, de Groot et al observe that when a glycan is attached to position 476 in the Cys-rich domain, binding of the ADAMTS13 variant to VWF and its proteolytic activity are significantly reduced (see panel B), suggesting the importance of this glycan attaching site and perhaps its vicinity for ADAMTS13 function. Second, by swapping the Cys-rich domain between ADAMTS13 and ADAMTS1, a closely related member of the ADAMTS family, they are able to identify a hydrophobic pocket in the Cys-rich domain involving residues Gly471-Val474 that is critical for VWF binding and proteolysis (see figure panel B). Third, in a reversed experiment, de Groot et al further identify a hydrophobic pocket comprising residues Ile1642, Trp1644, Ile1649, Leu1650, and Ile1651 in the central A2 domain of VWF as being part of the complementary site for interaction with the hydrophobic pocket involved in residues Gly471-Val474 in the Cys-rich domain of ADAMTS13. The findings in this study provide novel insight into the role of the Cys-rich domain and bridge a major gap in our understanding of the structural and functional relationship of ADAMTS13. However, the confirmation of such an interaction between ADAMTS13 and VWF relies on cocrystallization of the VWF peptide-ADAMTS13 enzyme complex or other more sophisticated biochemical/biophysical techniques. Whether anti-ADAMTS13 immunoglobulin G autoantibodies in acquired TTP patients bind to this hydrophobic pocket in the Cys-rich domain to inhibit ADAMTS13 proteolytic function remains to be determined.
Conflict-of-interest disclosure: The author declares no competing financial interests.