In this issue of Blood, Rollin et al demonstrate that heparin-induced thrombocytopenia and thrombosis (HIT) patients homozygous for arginine (R) at position 131 in the immunoglobulin (Ig)G receptor FcγRIIA have a significantly higher incidence of thrombosis, and show that plasma IgG may play a key role in modulating the thrombotic process.1
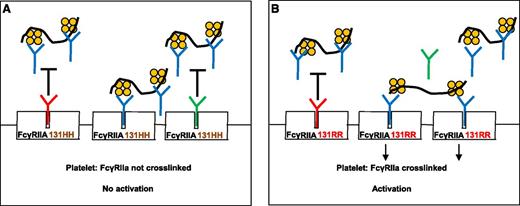
Rollin et al describe the role of IgG in modulating HIT-antibody–mediated platelet activation. (A) When plasma IgG1 (red “Y”) or IgG2 (green “Y”) bind to the platelet IgG receptor, FcγRIIA 131HH, HIT antibody (blue “Y”) binding to FcγRIIA is inhibited (black “T”). FcγRIIA is not crosslinked and platelets are not activated. (B) Plasma IgG1 binds effectively to FcγRIIA 131RR and inhibits HIT-antibody binding, but relatively more FcγRIIA receptors are left unoccupied because IgG2 binds poorly to 131RR. HIT antibodies can then bind to unoccupied receptors and crosslink them, resulting in platelet activation. The black squiggle denotes heparin or other glycosaminoglycans and the orange tetramer depicts PF4.
Rollin et al describe the role of IgG in modulating HIT-antibody–mediated platelet activation. (A) When plasma IgG1 (red “Y”) or IgG2 (green “Y”) bind to the platelet IgG receptor, FcγRIIA 131HH, HIT antibody (blue “Y”) binding to FcγRIIA is inhibited (black “T”). FcγRIIA is not crosslinked and platelets are not activated. (B) Plasma IgG1 binds effectively to FcγRIIA 131RR and inhibits HIT-antibody binding, but relatively more FcγRIIA receptors are left unoccupied because IgG2 binds poorly to 131RR. HIT antibodies can then bind to unoccupied receptors and crosslink them, resulting in platelet activation. The black squiggle denotes heparin or other glycosaminoglycans and the orange tetramer depicts PF4.
HIT, a major cause of morbidity in patients treated with heparin, is caused by Ig that recognize platelet factor 4 (PF4) in a complex with heparin.2 HIT is characterized by thrombocytopenia, often complicated by thrombosis. In this disorder, most of the morbidity stems from venous and arterial thrombosis, whereas bleeding due to thrombocytopenia is only rarely seen. Platelets express an IgG receptor, FcγRIIA, which when appropriately engaged by the Fc portion of HIT antibodies, activates platelets through signal transduction mediated by its intracellular immunoreceptor tyrosine-based activation motif. FcγRIIA has two codominantly expressed alleles with either histidine (H) or R in position 131 of its extracellular second Ig-like domain. IgG2 binds substantially better to FcγRIIA 131HH than it does to FcγRIIA 131RR.3 On the other hand, IgG1 binds equally well to the 131HH and RR FcγRIIA phenotypes.4
In a group of 84 HIT patients, Rollin et al found that the FcγRIIA 131RR genotype is significantly over-represented in the group of patients who developed thrombosis compared with the group who did not (34.5% vs 8.5%). No association between FcγRIIA 131RR and thrombosis was found in patient cohorts who had thrombosis not associated with HIT, suggesting that this association may be HIT-specific. Previous studies of this relationship have yielded conflicting results,5,6 but in one large study,5 an association between 131RR and thrombosis was found. Greater clearance of HIT immune complexes by the FcγRIIA 131HH receptor was proposed to explain this association.5 However, this is unlikely since the majority of HIT antibodies are of the IgG1 subclass.6 Rollin et al describe studies to provide an alternative explanation for the apparent increase in thrombotic risk in HIT patients who possess the FcγRIIA 131RR phenotype. For this work, they used HIT patient sera and a newly developed chimeric humanized anti-PF4/heparin monoclonal antibody, 5B9, which appears to mimic human HIT antibody behavior.
Platelet-activating HIT antibodies induce platelet aggregation when added to platelet-rich plasma (PRP) along with heparin at a low dose. In the study, platelet aggregation testing performed with PRP but not with washed platelets, demonstrated a significantly prolonged lag time when platelets from 131HH donors were used compared with 131RR and 131RH heterozygote donors. This difference was abrogated when IgG-depleted PRP was used. Performing the experiment after repletion of polyclonal IgG recapitulated the results obtained with PRP (ie, the lag time before aggregation was shorter with 131RR platelets than with 131HH platelets). When the experiment was performed with the addition of IgG1 alone (which binds equally well to FcγRIIA 131RR and 131HH), lag times of platelet aggregation from both 131RR and 131HH donors were comparable. However, when Ig repletion was done with IgG2, there was a striking increase in lag time only when platelets expressing the 131HH FcγRIIA isoform were used. The much lower affinity of IgG2 for FcγRIIA 131RR was invoked to explain this finding. Interestingly, higher tissue factor gene expression and higher phospholipid procoagulant activity were also observed when HIT antibody and low-dose heparin were added to whole blood samples from FcγRIIA 131RR donors than when whole blood from 131HH donors was used.
Modulation of HIT-antibody–induced platelet activation by normal plasma IgG as proposed in this interesting study (see figure) suggests a possible rationale for use of intravenous immunoglobulin (IVIg) to downregulate platelet activation by HIT antibodies. Numerous anecdotal reports7-9 suggest that this treatment can be efficacious in some HIT patients. If this benefit is confirmed in future studies, IVIg could supplement the few medications that are currently available to prevent and treat thrombosis in HIT.10 Also, if further studies confirm the findings of Rollin et al, FcγRIIA genotyping may be useful in thrombosis risk stratification of HIT patients, and conceivably, patients with the 131RR genotype may be candidates for more aggressive thromboprophylaxis.
Conflict-of-interest disclosure: The author declares no competing financial interests.