Key Points
Coadministering FIX orally and systemically induces tolerance via complex immune regulation, involving tolerogenic dendritic and T-cell subsets.
Induced CD4+CD25−LAP+ regulatory T cells with increased IL-10 and TGF-β expression and CD4+CD25+ regulatory T cells suppress antibody formation against FIX.
Abstract
Coagulation factor replacement therapy for the X-linked bleeding disorder hemophilia is severely complicated by antibody (“inhibitor”) formation. We previously found that oral delivery to hemophilic mice of cholera toxin B subunit-coagulation factor fusion proteins expressed in chloroplasts of transgenic plants suppressed inhibitor formation directed against factors VIII and IX and anaphylaxis against factor IX (FIX). This observation and the relatively high concentration of antigen in the chloroplasts prompted us to evaluate the underlying tolerance mechanisms. The combination of oral delivery of bioencapsulated FIX and intravenous replacement therapy induced a complex, interleukin-10 (IL-10)–dependent, antigen-specific systemic immune suppression of pathogenic antibody formation (immunoglobulin [Ig] 1/inhibitors, IgE) in hemophilia B mice. Tolerance induction was also successful in preimmune mice but required prolonged oral delivery once replacement therapy was resumed. Orally delivered antigen, initially targeted to epithelial cells, was taken up by dendritic cells throughout the small intestine and additionally by F4/80+ cells in the duodenum. Consistent with the immunomodulatory responses, frequencies of tolerogenic CD103+ and plasmacytoid dendritic cells were increased. Ultimately, latency-associated peptide expressing CD4+ regulatory T cells (CD4+CD25−LAP+ cells with upregulated IL-10 and transforming growth factor-β (TGF-β) expression) as well as conventional CD4+CD25+ regulatory T cells systemically suppressed anti-FIX responses.
Introduction
Inherited protein deficiencies are typically treated by IV administration of concentrates of functional recombinant protein. However, a major complication of these replacement therapies is antibody formation against infused therapeutic antigen. This is well documented for the X-linked bleeding disorder hemophilia, which is caused by deficiency of coagulation factor VIII (hemophilia A) or factor IX (FIX, hemophilia B). Severe disease (<1% coagulation activity) typically results in frequent spontaneous and potentially life-threatening bleeding, causing disability, pain, and reduced quality of life. Neutralizing antibodies, termed “inhibitors,” form in 20% to 30% of severe hemophilia A patients, thereby substantially complicating and increasing costs of treatment.1 Although inhibitors form less frequently in hemophilia B (∼5% of severe patients), they tend to be high titer and are associated with anaphylactic reactions against FIX in ≥25% of cases.2 Clinical immune tolerance induction protocols (daily high-dose factor administration) are lengthy (months to >1 year), expensive, and are often terminated in hemophilia B because of anaphylaxis or nephrotic syndrome. Alternative approaches are desirable. In particular, there are currently no prophylactic immune tolerance protocols.
Because of easy administration, antigen specificity, and lack of toxicity, oral tolerance has long been discussed as a potentially ideal method to prevent inhibitor formation.1,3 The intestinal immune system is routinely exposed to a large variety of antigens, including dietary proteins and constituents of commensal bacteria. Importantly, the gut immune system has evolved tightly regulated mechanisms to suppress unwanted inflammatory responses, while still protecting from pathogenic organisms.4,5 It was hypothesized that ingested coagulation factor would prevent systemic responses during replacement therapy. However, inability to cost-effectively produce and to adequately deliver coagulation factors to the gut immune system kept this concept from becoming reality.3 Low levels of antigen expression had previously limited the use of transgenic crop plants for oral tolerance, which would avoid costly purification methods. Taking advantage of the high number of chloroplast genomes per cell, we overcame these hurdles with our optimized technology for chloroplast transformation and gene expression.6 Oral administration of factor VIII or FIX antigens expressed in transplastomic tobacco plants suppressed inhibitor formation and anaphylaxis in hemophilic mice.7,8 A combination of protection from digestion offered by bioencapsulation in plant cells and fusion to the transmucosal carrier cholera toxin B (CTB subunit, thereby targeting gut epithelial cells) resulted in efficient tolerogenic delivery. Surprisingly little is known about the mechanism of oral tolerance induction of antigens expressed in plant cells, such as the role of antigen-presenting cells (APCs) or regulatory T cells (Tregs). Our recent data support earlier literature that active suppression occurs.7,9
Here, we demonstrate that plant cell-based oral tolerance for hemophilia is the result of a complex immune regulatory mechanism in response to the combination of orally and systemically administered antigen, ultimately inducing 2 types of Treg that suppress antibody formation. Immune suppressive cytokines transforming growth factor-β (TGF-β) and interleukin-10 (IL-10) are key factors in the orchestration of this tolerogenic response.
Materials and methods
Chloroplast transgenic plant material
Transplastomic tobacco plants with stable integration of a human FIX-CTB fusion construct in the chloroplast genome were as published.8 A furin cleavage site and a glycine-proline-glycine-proline hinge is present between the CTB and FIX fusion elements. Transgene expression is regulated by the psbA promoter and 5′ untranslated region.8 Leaf material was harvested for oral delivery, ground in liquid nitrogen, and stored at −80°C.7,8 FIX-CTB antigen levels (in microgram/gram total frozen leaf material) were determined by western blot.8
Animal experiments
Hemophilia B C3H/HeJ mice (F9 gene deletion) were as published.8,10-13 Male mice approximately 2 months of age were used at the onset of experiments and housed under special pathogen-free conditions. Plant material (125 mg per dose) was suspended in 200 μL of sterile phosphate-buffered saline, homogenized, and orally delivered via gavage using a 20-G bulb-tipped gastric gavage needle. For FIX challenge, mice were administrated 1 IU hFIX (Benefix, Pfizer, New York, NY) into the tail vein once per week. Blood collection by tail bleed was as described previously.10 C3H/HeJ Il10−/− mice were obtained from Jackson Laboratories and crossed with hemophilia B mice to obtain C3H/HeJ F9−/− × Il10−/− mice.
T-cell preparation and adoptive transfer
Spleens and mesenteric lymph nodes (MLNs) were harvested, homogenized, and treated with red blood cell lysis buffer (Sigma, St. Louis, MO). T-cell subsets were isolated via magnetic sorting methods using the Treg isolation and CD4+ T-cell isolation kits from Miltenyi Biotech (Auburn, CA), and anti-mouse latency-associated peptide (LAP)-phycoerythrin (eBiosciences, San Diego, CA) according to manufacturer’s protocols. Purity of the cells was above 90% as confirmed by flow cytometry. Purified live cells as determined by trypan blue staining (1 × 106 per mouse) were adoptively transferred into naive strain-matched mice via tail vein injection. After 24 hours, recipient mice were subcutaneously injected with 1 IU FIX formulated in Sigma Adjuvant System. Blood was collected 3 weeks later.
Antibody assays
FIX-specific immunoglobulins in plasma were measured by enzyme-linked immunosorbent assay and, to determine inhibitor titers, by Bethesda assay as published.8,10,11 By definition, 1 Bethesda unit (BU) indicates the degree of inhibition of 50% residual coagulation activity. For stool samples, FIX-specific and total fecal immunoglobulin (Ig)A were determined. Fresh fecal pellets were diluted 1:2 (wt/vol) in phosphate-buffered saline/0.1% Tween 20 containing a complete proteinase inhibitor cocktail (Invitrogen). Samples were briefly vortexed and centrifuged twice at 13 000g for 10 minutes, and supernatants stored at −80°C.
Antibody stains and gene expression profiling
Antibody stains for flow cytometry and immunohistochemistry were performed using standard methods (see supplemental Materials, available on the Blood Web site for details). For gene expression profiling, single-cell suspension of splenocytes derived from FIX-fed and control mice were cultured ± stimulation with 10 μg/mL FIX.7,11 After 48 hours, messenger RNA was extracted, reverse transcribed, and analyzed by quantitative polymerase chain reaction (PCR) using primer-precoated 96-well plates (SABiosciences).11
Statistics
Differences between the 2 experimental groups were compared by unpaired 2-tailed Student t test. The differences among several groups were compared by 2-way analysis of variance. Differences in survival rates were examined by the log-rank test. All analyses were performed with SAS version 9.3 and were considered significant for P < .05.
Results
Oral administration of FIX transgenic plant leaves modulates antibody responses against intravenous FIX in hemophilia B mice
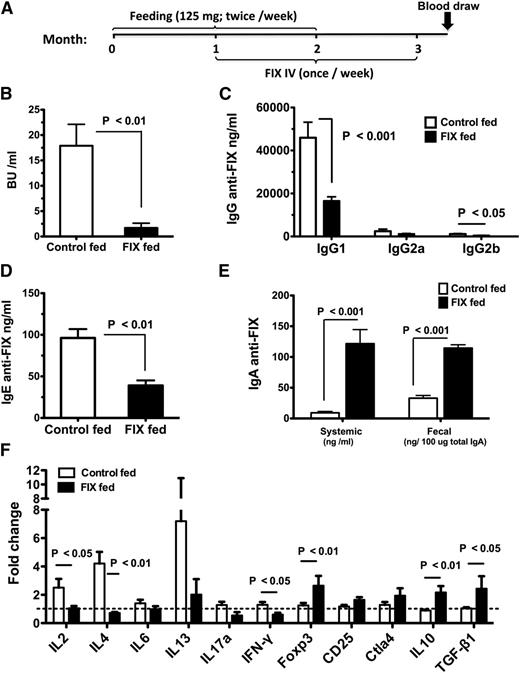
Previously, we found that a wide range of FIX antigen doses contained in transplastomic tobacco cells was effective in suppressing inhibitor formation and anaphylaxis against intravenously delivered recombinant human FIX.8 Here, we used 125 mg frozen transgenic plant cells expressing CTB-FIX fusion protein in chloroplasts, containing an intermediate dose of 5 μg for oral gavage of hemophilia B mice with F9 gene deletion (C3H/HeJ F9−/−), twice per week for 2 months (Figure 1A). Mice were challenged with 1 IU FIX IV once per week for 2 months, starting 1 month after onset of oral gavages. Control mice were gavaged with untransformed wild-type (WT) tobacco cells. To prevent fatal anaphylaxis in control animals, antihistamine and antiplatelet activating factor (anti-PAF) were given starting at the 4th injection.8,11 Consistent with our previous data, inhibitor/IgG1 formation (the predominant IgG formed against FIX) and also IgE formation was markedly suppressed (Figure 1B-D).8 Only 1/8 tolerized mice developed an inhibitor of >5 BU (with 4/8 mice showing entirely undetectable titers), whereas 7/8 control mice had >5 BU (up to 37 BU). Suppression of inhibitor formation resulted in improved correction of coagulation by intravenously injected FIX (supplemental Figure 1). No signs of anaphylaxis were observed in the tolerized mice despite not having received antihistamine/anti-PAF. Low systemic and secreted IgA anti-FIX was induced in tolerized mice (Figure 1E), which does not prevent long-term oral FIX delivery however (published data).8 No suppression of antibody formation against a different antigen was observed in FIX-tolerized mice, supporting antigen specificity (supplemental Figure 2).14 Finally, no antibody formation (IgG or IgA) against CTB was detected (data not shown).
Modulation of antibody formation by oral administration of bioencapsulated FIX in hemophilia B mice. (A) Timeline and schedule for oral gavages (FIX or WT plant material, n = 8 per experimental group), IV FIX administration, and blood collection for antibody assays. (B) Inhibitor titers (in BU/mL plasma). (C) FIX-specific IgG titers (IgG1, IgG2a, and IgG2b (ng/mL). (D) FIX-specific IgE titers (ng/mL) and (E) FIX-specific IgA titers (in ng/mL for circulating IgA and in ng/100 μg total IgA for fecal titers). (F) At the end of the experiment, in vitro splenocyte cultures were established. Cells were stimulated with or without 10 μg/mL FIX antigen for 48 hours, followed by quantitative RT-PCR analysis. “Fold increase” is change in RNA transcripts of FIX vs mock stimulated. All gene expression was compared with that of glyceraldehyde-3-phosphate dehydrogenase. Fold-change was calculated using the 2ΔΔCt quantification method. The dotted horizontal line indicates 1-fold change. (B-F) Average ± standard error of the mean (SEM).
Modulation of antibody formation by oral administration of bioencapsulated FIX in hemophilia B mice. (A) Timeline and schedule for oral gavages (FIX or WT plant material, n = 8 per experimental group), IV FIX administration, and blood collection for antibody assays. (B) Inhibitor titers (in BU/mL plasma). (C) FIX-specific IgG titers (IgG1, IgG2a, and IgG2b (ng/mL). (D) FIX-specific IgE titers (ng/mL) and (E) FIX-specific IgA titers (in ng/mL for circulating IgA and in ng/100 μg total IgA for fecal titers). (F) At the end of the experiment, in vitro splenocyte cultures were established. Cells were stimulated with or without 10 μg/mL FIX antigen for 48 hours, followed by quantitative RT-PCR analysis. “Fold increase” is change in RNA transcripts of FIX vs mock stimulated. All gene expression was compared with that of glyceraldehyde-3-phosphate dehydrogenase. Fold-change was calculated using the 2ΔΔCt quantification method. The dotted horizontal line indicates 1-fold change. (B-F) Average ± standard error of the mean (SEM).
Inhibitor formation is thought to be dependent on T-helper cells.1 To investigate whether suppression of anti-FIX formation reflected altered T-cell responses, we performed reverse transcriptase (RT)-PCR array analysis on splenocytes that had been in vitro–stimulated with FIX. As shown in Figure 1F, control mice showed expression of IL-2, interferon-γ, IL-4, and IL-13 cytokines, all of which were suppressed in tolerized mice, which instead upregulated molecules associated with immune suppression, in particular transcription factor FoxP3 and cytokines TGF-β and IL-10.
Increases in frequencies of tolerogenic DCs, CD4+CD25−LAP+ T cells, and gut homing receptor expressing CD4+ T cells after IV challenge with FIX
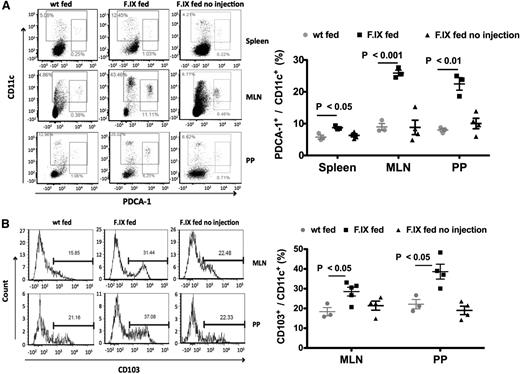
Oral tolerance requires function of specific dendritic cells (DCs). For example, plasmacytoid DCs (pDCs) can transfer oral tolerance in animal models and likely contribute to T-cell tolerance.15 CD103+CD11c+ DCs acquire antigen in the small intestine and migrate to draining lymph nodes (such as the MLNs) to activate Treg and induce their migration to the lamina propria (LP), where they are further expanded in the presence of the highly enriched CX3CR1+CD11b+CD11c+ DCs.16 Here, the combination of oral and systemic FIX delivery (but not oral delivery by itself) caused a significant increase in the frequencies and numbers of pDCs in spleen, MLNs, and Peyer patches (PP) (Figure 2A) as well as of CD103+ DCs in MLNs and PPs (supplemental Figures 2B, 3, and 4).
Increases of tolerogenic DCs in hemophilia B mice. (A) Flow cytometry analysis of CD11c and PDCA-1 staining in the spleen, MLNs, and PPs in hemophilia B mice following oral delivery of FIX plant material and IV challenge with FIX. Control groups were fed with WT plant material (wt fed) or with FIX material but not challenged with IV injections of FIX (FIX fed no injection). (B) CD103 and CD11c staining of MLN and PP cells of the same mice. Data points are for individual mice. Averages ± SEM are also indicated (n = 3-5 per experimental group). Shown are data from 2 independent experiments that showed similar results.
Increases of tolerogenic DCs in hemophilia B mice. (A) Flow cytometry analysis of CD11c and PDCA-1 staining in the spleen, MLNs, and PPs in hemophilia B mice following oral delivery of FIX plant material and IV challenge with FIX. Control groups were fed with WT plant material (wt fed) or with FIX material but not challenged with IV injections of FIX (FIX fed no injection). (B) CD103 and CD11c staining of MLN and PP cells of the same mice. Data points are for individual mice. Averages ± SEM are also indicated (n = 3-5 per experimental group). Shown are data from 2 independent experiments that showed similar results.
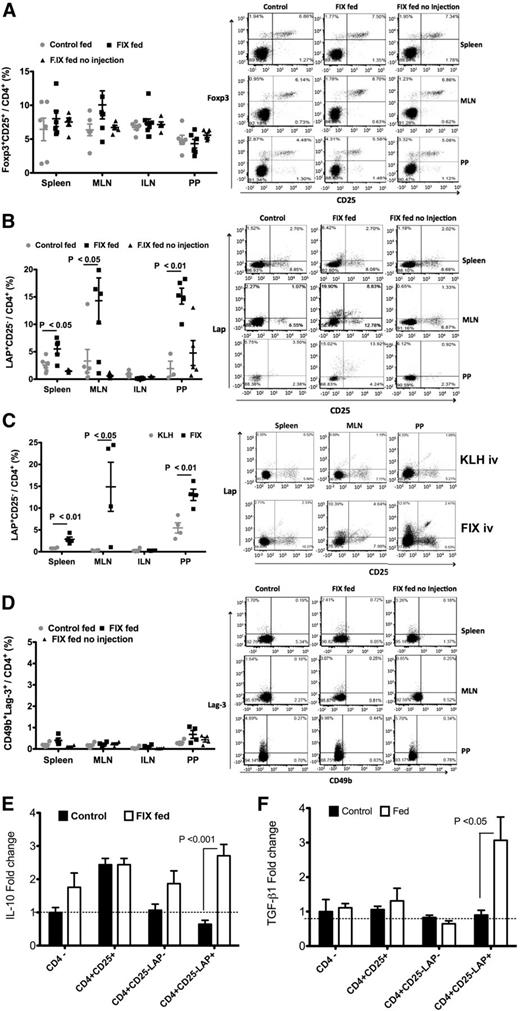
When examining different subsets of Treg, minimal changes in the overall frequencies/numbers of CD4+CD25+FoxP3+ Treg or type 1 regulatory T cells (Tr1 cells, defined as CD4+LAG-3+CD49b+) were observed in the spleen, MLNs, or PP (Figure 3A,D; supplemental Figure 5). CD4+CD25−FoxP3− T cells suppressing by a TGF-β–dependent mechanism were initially termed Th3 cells,4 but are now better defined by their expression of a membrane bound form of TGF-β, containing the amino-terminal domain LAP.17 We found that CD4+CD25−LAP+ cells were significantly increased in the spleen and even more robustly in PP and MLNs (but not in nondraining inguinal lymph nodes). This was only seen after IV challenge with FIX (Figure 3B), but not with a different antigen, thus representing an antigen-specific response (Figure 3C).4 Analysis of cytokine expression in different cell types of FIX-stimulated lymphocytes showed that CD4+CD25−LAP+ Tregs were the main source of increased IL-10 and TGF-β expression in FIX-fed/systemically treated mice (Figure 3E,F). In addition, there was an increase in LAP+ CD4+CD25+FoxP3+ Tregs in PP (supplemental Figure 6), indicating activation of FoxP3+ Treg. Tr1 cells also highly express IL-10, which has a critical anti-inflammatory role and is abundantly produced by LP lymphocytes.18 Upon examination of this compartment, we found a modest increase in the frequency of Tr1 cells, which upregulated IL-10 expression in response to FIX (supplemental Figure 7).
Changes in Treg frequencies in hemophilia B mice. Flow cytometric analyses of spleen, MLN, ILN, and PP cells of hemophilia B mice following oral delivery of FIX plant material and IV challenge with FIX. Control groups were fed with WT plant material (wt fed) or were fed with FIX material but not challenged with IV injections of FIX (FIX fed no injection). (A) CD4+CD25+FoxP3+ Tregs; (B,C) CD4+CD25−LAP+ Tregs; and (D) Tr1 cells (CD49b+LAG-3+CD4+). (C) Independent experiment comparing the CD4+CD25−LAP+ Treg frequencies of mice that were fed with FIX and intravenously challenged either with FIX or with and identical antigen dose of keyhole limpet hemocyanin (KLH). Data points are for individual mice. Averages ± SEM are also indicated (n = 3-6 per group). Representative flow cytometry plots are shown for spleen, MLNs, and PPs. (E,F) Quantitative RT-PCR analysis of IL-10 (E) and TGF-β (F) expression in different subsets of magnetically sorted cells from FIX-fed and control mice after a 48-hour lymphocyte culture established for individual animals (and comprising pooled MLN cells and splenocytes). Data indicate average cytokine expression levels (± SEM) for FIX-stimulated relative to unstimulated cultures (n = 6 mice/cell type, except for CD4+CD25+ cells, which was n = 3).
Changes in Treg frequencies in hemophilia B mice. Flow cytometric analyses of spleen, MLN, ILN, and PP cells of hemophilia B mice following oral delivery of FIX plant material and IV challenge with FIX. Control groups were fed with WT plant material (wt fed) or were fed with FIX material but not challenged with IV injections of FIX (FIX fed no injection). (A) CD4+CD25+FoxP3+ Tregs; (B,C) CD4+CD25−LAP+ Tregs; and (D) Tr1 cells (CD49b+LAG-3+CD4+). (C) Independent experiment comparing the CD4+CD25−LAP+ Treg frequencies of mice that were fed with FIX and intravenously challenged either with FIX or with and identical antigen dose of keyhole limpet hemocyanin (KLH). Data points are for individual mice. Averages ± SEM are also indicated (n = 3-6 per group). Representative flow cytometry plots are shown for spleen, MLNs, and PPs. (E,F) Quantitative RT-PCR analysis of IL-10 (E) and TGF-β (F) expression in different subsets of magnetically sorted cells from FIX-fed and control mice after a 48-hour lymphocyte culture established for individual animals (and comprising pooled MLN cells and splenocytes). Data indicate average cytokine expression levels (± SEM) for FIX-stimulated relative to unstimulated cultures (n = 6 mice/cell type, except for CD4+CD25+ cells, which was n = 3).
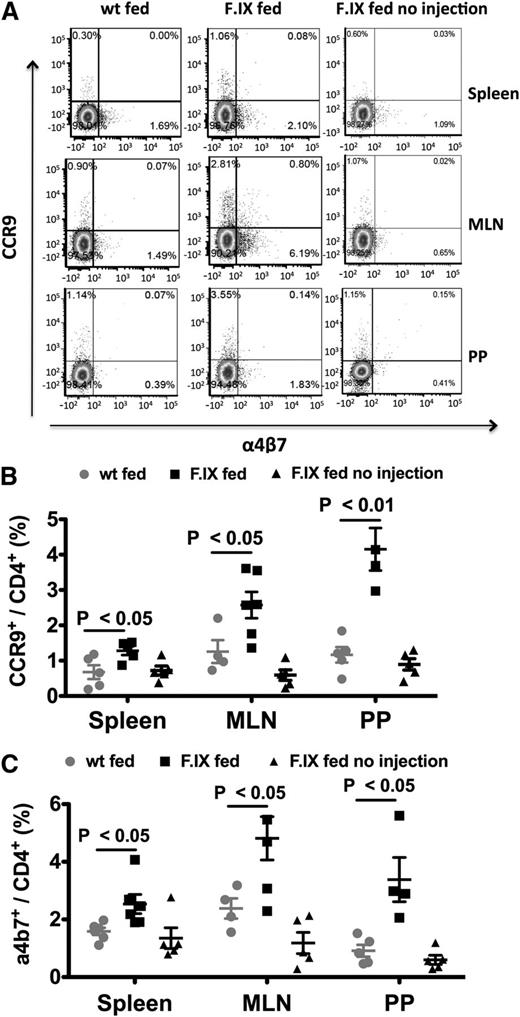
DCs from MLNs, PP, and small intestine LP were previously reported to induce high expression of gut-homing receptors integrin α4β7 and chemokine receptor CCR9 on lymphocytes.19,20 Gut-homing T cells are critical for oral tolerance induction. For example, expression of gut homing receptors causes Treg to egress the MLNs and home toward the small intestine LP, instead of entering directly into the bloodstream.19,20 The oral tolerance regimen followed by IV challenge significantly increased frequencies of α4β7 and CCR9 expressing CD4+ T cells in MLNs, PP, and also the spleen, illustrating a linked local (gut) and systemic T-cell response (Figure 4A-C).
Increases of gut-homing receptor expressing CD4+ T cells in hemophilia B mice. (A) Representative examples of flow cytometric analysis of CCR9 and integrin α4β7 staining. Frequencies of CCR9 (B) and α4β7 (C) expressing cells in the spleen, MLNs, and PPs following oral delivery of FIX plant material and IV challenge with FIX. Control groups were fed with WT plant material (wt fed) or with FIX material but not challenged with IV injections of FIX (FIX fed no injection). Data points are for individual mice, with average ± SEM also indicated (n = 4-6 per group).
Increases of gut-homing receptor expressing CD4+ T cells in hemophilia B mice. (A) Representative examples of flow cytometric analysis of CCR9 and integrin α4β7 staining. Frequencies of CCR9 (B) and α4β7 (C) expressing cells in the spleen, MLNs, and PPs following oral delivery of FIX plant material and IV challenge with FIX. Control groups were fed with WT plant material (wt fed) or with FIX material but not challenged with IV injections of FIX (FIX fed no injection). Data points are for individual mice, with average ± SEM also indicated (n = 4-6 per group).
Induction of CD4+CD25+ and CD4+CD25−LAP+ Tregs that suppress anti-FIX formation
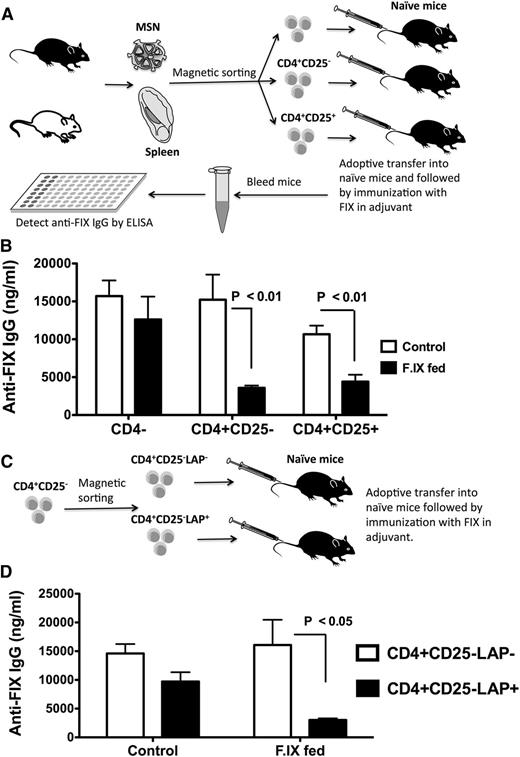
To demonstrate induction of Tregs that suppress antibody formation against FIX in vivo and to identify subsets of Tregs that mediate suppression, we sorted 3 populations of cells from MLNs and spleens of hemophilia B mice that received the oral tolerance/IV challenge regimen. CD4−, CD4+CD25+, and CD4+CD25− cells were adoptively transferred to naive mice of the same strain, followed by immunization with FIX (Figure 5A). Control mice received cells from naive hemophilia B mice (to account for nonspecific suppression). Both CD4+CD25+ and CD4+CD25− (but not CD4−) cells from tolerized mice similarly suppressed anti-FIX formation (Figure 5B).
Plant-based oral tolerance induces CD4+CD25+ Tregs and LAP+CD25−CD4+ Tregs. (A) Experimental outline of adoptive transfer. CD4−, CD4+CD25−, and CD4+CD25+ cells were purified via magnetic sorting from spleens and MLNs of FIX-fed/intravenously challenged hemophilia B mice and pooled. Doses of 106 cells per mouse were adoptively transferred into strain-matched mice via tail vein injection. Control cells were from naive mice. After 24 hours, all recipient mice (n = 5-8 per group) were challenged with 1 IU FIX in adjuvant via subcutaneous injection. IgG titers against FIX (B) were determined 3 weeks later. (C) Purified CD4+CD25− cells were further divided into LAP+ and LAP− cells via magnetic sorting. The cells were adoptively transferred (n = 4-6 per group), followed by immunization with FIX. IgG titers against FIX (D) were determined 3 weeks later. Control mice had received cells from naive mice. Data are average ± SEM. Shown are data from 2 independent experiments that showed similar results.
Plant-based oral tolerance induces CD4+CD25+ Tregs and LAP+CD25−CD4+ Tregs. (A) Experimental outline of adoptive transfer. CD4−, CD4+CD25−, and CD4+CD25+ cells were purified via magnetic sorting from spleens and MLNs of FIX-fed/intravenously challenged hemophilia B mice and pooled. Doses of 106 cells per mouse were adoptively transferred into strain-matched mice via tail vein injection. Control cells were from naive mice. After 24 hours, all recipient mice (n = 5-8 per group) were challenged with 1 IU FIX in adjuvant via subcutaneous injection. IgG titers against FIX (B) were determined 3 weeks later. (C) Purified CD4+CD25− cells were further divided into LAP+ and LAP− cells via magnetic sorting. The cells were adoptively transferred (n = 4-6 per group), followed by immunization with FIX. IgG titers against FIX (D) were determined 3 weeks later. Control mice had received cells from naive mice. Data are average ± SEM. Shown are data from 2 independent experiments that showed similar results.
Although induction of CD4+CD25+ Treg seems generally critical for immune tolerance induction to FIX, CD4+CD25− had not been found to be suppressive in other protocols.11,12,21-33 To further define this additional subset of Treg induced by oral tolerance, we repeated the adoptive transfer of CD4+CD25−, which we now divided into LAP+ and LAP− cells (Figure 5C; supplemental Figure 8). Only CD4+CD25−LAP+ cells were found to specifically suppress anti-FIX formation (Figure 5D; supplemental Figure 9).
Uptake of fed FIX antigen in the small intestine
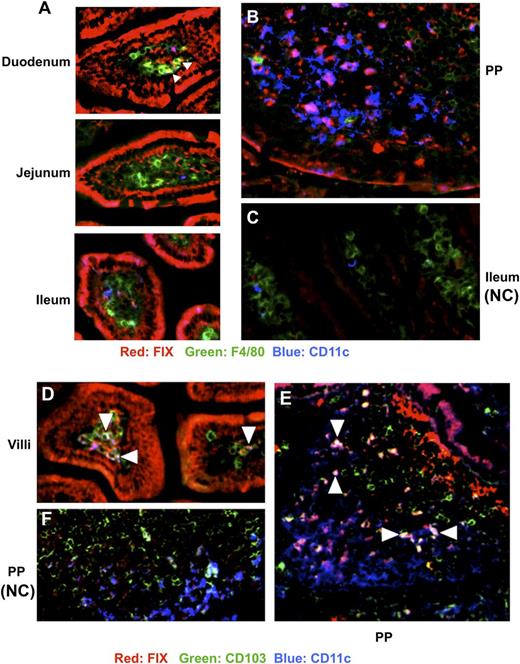
Antigens are mainly sampled and processed in the intestine by mononuclear phagocytes, including macrophages and DCs, which are critical for differentiation, expansion, and maintenance of Tregs.34 The plant-expressed FIX antigen is fused with CTB, which exhibits specific high-affinity binding to the oligosaccharide domain of GM1 ganglioside. Hence, epithelial cells of fed animals stained widely positive for FIX throughout the ileum using immunohistochemistry (red stain, Figure 6A,D). We found examples of FIX+CD11c+ (purple) and FIX+CD103+CD11c+ (white/light blue) DCs in villi and PP throughout the ileum in different regions, whereas FIX+ cells expressing the macrophage marker F4/80 were only found in villi of the upper part of the ileum (yellow cells, duodenum; Figure 6A,B,E and data not shown; control sections from animals that had not received FIX antigen are shown in Figure 6C,F). In areas of delivery to DCs, CD103+ made up 37% to 60% of FIX+CD11c+ cells. Consistent with our previous findings, CTB was found translocated across the epithelial cell barrier and did not colocalize with FIX (supplemental Figure 10A), presumably because of efficient furin cleavage at the site between FIX and CTB.35 Stains of the spleen showed positivity for FIX but not CTB (supplemental Figure 10B), which is expected because CTB is retained intracellularly, whereas cleaved FIX is in part systemically delivered.8,35
Uptake of luminal FIX antigen by APCs in the small intestine. Immunohistochemistry of small intestine of hemophilia B mice. (A) Representative sections of villi from duodenum, jejunum, ileum, or (B) PP stained with antibody against FIX (red), F4/80 (green), and CD11c (blue) of mice fed with CTB-FIX expressing plant material. (C) Same stain of villi from ileum of a control mouse fed with WT material (NC, negative control). (D) Staining with antibody against FIX (red), CD103 (green), and CD11c (blue) of villi or (E) PPs from a mouse fed with CTB-FIX expressing or (F) WT plant material. Triangles in panel A point to yellow FIX+F4/80+ cells (yellow). Purple cells in panels A, B, and D are FIX+CD11c+ DCs. White/light blue cells in panels D and E are FIX+CD11c+CD103+ DCs (examples are indicated with triangles).
Uptake of luminal FIX antigen by APCs in the small intestine. Immunohistochemistry of small intestine of hemophilia B mice. (A) Representative sections of villi from duodenum, jejunum, ileum, or (B) PP stained with antibody against FIX (red), F4/80 (green), and CD11c (blue) of mice fed with CTB-FIX expressing plant material. (C) Same stain of villi from ileum of a control mouse fed with WT material (NC, negative control). (D) Staining with antibody against FIX (red), CD103 (green), and CD11c (blue) of villi or (E) PPs from a mouse fed with CTB-FIX expressing or (F) WT plant material. Triangles in panel A point to yellow FIX+F4/80+ cells (yellow). Purple cells in panels A, B, and D are FIX+CD11c+ DCs. White/light blue cells in panels D and E are FIX+CD11c+CD103+ DCs (examples are indicated with triangles).
To test for uptake of systemically delivered protein antigen in the gut, Alex Fluor 647–labeled ovalbumin was intravenously injected and found to be taken up by F4/80+ and CD11c+ cells in the ileum (supplemental Figure 11).36
IL-10 is required for oral tolerance induction
IL-10 signaling is required for Treg to maintain the suppressive function on mucosal interphases and has critical anti-inflammatory functions in the gut.37 Therefore, we wanted to test for a requirement of IL-10 in our model. IL-10–deficient hemophilia B mice were generated by crossing C3H/HeJ Il10−/− with C3H/HeJ F9−/− mice. The identical oral tolerance/challenge regimen was performed (supplemental Figure 12A), except that no pharmacological blockers of anaphylaxis were used. Both FIX-fed and control animals showed anaphylaxis, with identical survival rates (supplemental Figure 12B). Among the surviving animals, there was a minor nonsignificant reduction in inhibitor titers and IgG1 formation in FIX-fed mice (supplemental Figure 12C,D). Oral delivery of FIX failed to induce any of the changes in Treg and DC frequencies observed in IL-10+/+ hemophilia B mice (compare Figures 2-4 and supplemental Figure 13A-F).
Oral tolerance desensitizes preimmune mice but requires continuous feeding upon intravenous rechallenge with FIX
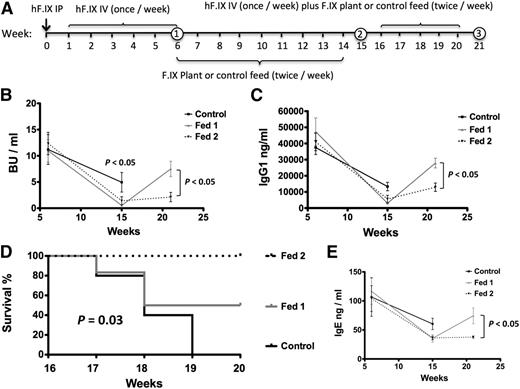
We next tested whether oral tolerance would be effective in animals with established inhibitors. As outlined in Figure 7A, C3H/HeJ F9−/− mice were first given FIX protein therapy for 6 weeks (with antihistamine/anti-PAF). The mice were then divided into 3 groups with similar average Bethesda titers (10-12 BU/mL). Control mice were fed with WT plant material (control, n = 5). A second group received FIX transgenic plant material during weeks 6 through 14 followed by WT plant material (fed 1, n = 6), and a third group received FIX transgenic leaves for weeks 6 through 20 (fed 2, n = 4). In all 3 groups, FIX IV therapy was resumed during weeks 16 through 20 (without antihistamine/anti-PAF). Although some modest decline in Bethesda titers occurred in the control group by week 15 (nonsignificant), oral gavage accelerated this decline substantially (from an average of 11 ± 5.3 BU/mL to 0.5 ± 0.4 BU/mL in fed 1, P < .001; from 12.4 ± 7 BU/mL to 1.4 ± 2.6 BU/mL in fed 2, P < .001; Figure 7B). IgG1 antibody responses dropped significantly in all 3 groups, albeit again more so in FIX-fed mice (Figure 7C). Upon continuation of FIX IV therapy, all control mice died of anaphylaxis, whereas the fed 1 group showed 50% survival, compared with 100% survival among fed 2 mice (Figure 7D). In surviving fed 1 mice, inhibitor/IgG1 and IgE titers rebounded, whereas antibody titers remained low in the fed 2 group (Figure 7B,C,E). Therefore, the protocol is effective in reversal of inhibitors and desensitization from anaphylaxis, but requires prolonged oral delivery when therapy is continued.
Reversal of inhibitor formation and anaphylaxis in hemophilia B mice. (A) Feeding and FIX administration schedule. Numbers in circle indicate time point of blood collection. (B) Inhibitor titers in BU/mL, (C) IgG1 titers against FIX at weeks 6, 15, and 21 of the experiment. Control mice were fed with WT plant material (control, black squares, n = 5). A second group received FIX transgenic plant material during weeks 6 to 14 followed by WT plant material (fed 1, gray triangles, n = 6), and a third group received FIX transgenic plant material for weeks 6 through 20 (fed 2, black triangles, n = 4). Data are average ± SEM. (D) Survival analysis in the same set of experimental mice. (E) IgE formation against FIX in plasma of the same mice.
Reversal of inhibitor formation and anaphylaxis in hemophilia B mice. (A) Feeding and FIX administration schedule. Numbers in circle indicate time point of blood collection. (B) Inhibitor titers in BU/mL, (C) IgG1 titers against FIX at weeks 6, 15, and 21 of the experiment. Control mice were fed with WT plant material (control, black squares, n = 5). A second group received FIX transgenic plant material during weeks 6 to 14 followed by WT plant material (fed 1, gray triangles, n = 6), and a third group received FIX transgenic plant material for weeks 6 through 20 (fed 2, black triangles, n = 4). Data are average ± SEM. (D) Survival analysis in the same set of experimental mice. (E) IgE formation against FIX in plasma of the same mice.
Discussion
Oral tolerance is particularly attractive because it does not rely on use of immune suppressive drugs, genetic manipulations of patient cells, expensive cell therapies, or invasive procedures. Recent clinical progress includes reversal of allergies—for example, using ground peanut material or transgenic rice seeds.4,38,39 Edible crop plants are potentially ideal for oral tolerance because they can be cost-effectively grown and provide natural encapsulation for the antigen through the plant cell wall without a need for protein purification, cold chain, or sterile injections.6 However, an oral tolerance protocol that could prevent antibody responses in protein replacement therapies for genetic disease had been elusive until recently. We developed chloroplast transgenic plants expressing high levels of coagulation factor antigens in green leaves and obtained substantial suppression of inhibitor formation in hemophilia A and B mice.7,8 Although these proof-of-principle studies used tobacco plants, transplastomic lettuce plants have now been generated for translational studies.40,41
High levels of antigen expression in chloroplasts facilitated this new study, which sought to define the mechanism of immune modulation by orally delivered plant-derived antigen. We demonstrate induction of a complex, IL-10–dependent and antigen-specific immune regulatory mechanism that reflects an interaction of the responses to ingested and systemically delivered FIX antigen. Release of FIX from plant cells is acquired by tolerogenic DCs in the small intestine. When combined with systemic replacement therapy, CD103+ DC and pDC numbers increase in various compartments, and several types of Tregs are induced. CD4+CD25−LAP+ Treg with FIX-specific upregulation of immune suppressive cytokines IL-10 and TGF-β constitute the largest component of the resulting systemic suppressive response, albeit induced CD4+CD25+ Treg are similarly capable of suppressing anti-FIX formation upon adoptive transfer. These results have multiple implications for further development of the approach and for tolerance induction in hemophilia in general. Overlap between oral delivery and systemic replacement therapy is important to achieve a strong Treg response, which is also critical for maintaining suppression of inhibitor formation in the long term,7,8 and for inhibitor reversal/desensitization from anaphylaxis. For the latter, prolonged overlap with oral delivery is critical when systemic delivery is resumed. Codelivery of IL-10 with the antigen to the gut immune system may further enhance Treg induction. Development of strategies that induce and/or use LAP+ Tregs (as an alternative to CD4+CD25+ Tregs) for suppression of inhibitor formation are warranted, and alternative oral tolerance regimens should be optimized for LAP+ Treg induction.
Multiple types of immune cells are required for oral tolerance, including pDCs, CD103+ DCs, and gut-homing receptors expressing CD4+ T cells,5 all of which were increased in our protocol. Two important subsets of gut-resident APCs have recently been characterized based on their nonoverlapping expression of CD103 (αE integrin) and CX3CR1 (fractalkine receptors). CD103+CD11c+ DCs derive from a blood precursor and home to the LP of the small intestine, MLN, and PP.16 They promote α4β7 and CCR9 expression on T cells, induce Foxp3+ Treg (via indoleamine 2,3-dioxygenase, retinoic acid, and TGF-β), and contribute to IgA+ B cell differentiation.4,16 We indeed observed IgA formation against FIX, which may occur independently of oral tolerance, such as in immune exclusion of pathogens.42 However, IgA also has anti-inflammatory properties and thus may contribute to tolerance.43 Upon migration from LPs to MLNs, CD103+CD11c+ exerts tolerogenic antigen presentation to T cells. F4/80+CX3CR1+ cells are less migratory but send protrusions into the gut lumen and are efficient in capture of soluble antigen, which is critical for the expansion of Tregs in the LPs and can be transferred to CD103+ DCs via gap junctions.34 Additionally, these cells acquire circulatory antigen (whereas CD103+ DCs mainly acquire fed antigen), and thus provide an important link between immune responses to systemic and ingested antigens.34,36 Consistent with these observations, F4/80+ cells took up plant-derived antigen in the upper part of the small intestine, while showing more widespread uptake of intravenously injected antigen.34 In contrast, CD103+ DCs took up orally delivered FIX in LPs and PPs throughout the small intestine. Two tolerogenic pathways of antigen presentation likely coexist. The same CD103+ DCs may present ingested (taken up directly or acquired from a F4/80+CX3CR1+ cell) and systemically delivered FIX antigen (acquired from a F4/80+CX3CR1+ cell). Alternatively, CD103+ DCs may present ingested antigen and induce Tregs that suppress antibody formation upon interaction with other APCs.
Bioencapsulated antigens are protected from stomach acids and enzymes, but are released to the immune system in the gut.6-8,14,35 Although the presence of microbes capable of lysing plant cells in the small intestine has been debated, it appears unlikely that the widespread delivery of FIX antigen that we observed could have been possible without a contribution of such commensal bacteria that digest plant cell walls. GM1 receptors are widely distributed over the intestinal mucosa with a rapid turnover rate.44 Chloroplast-expressed FIX-CTB forms the pentameric structure required for binding to GM1 gangliosides on intestinal epithelial cells, thereby allowing for endocytosis.8,45 Antigen-CTB-GM1 complexes then traffic retrograde through the Trans-Golgi network into the lumen of the endoplasmic reticulum.35,46-48 Because of the furin cleavage site, proteolytic cleavage occurs in the Trans-Golgi network, so that FIX antigen can be exocytosed and released into extracellular fluid, whereas CTB is retained intracellularly.8,35,46 The orally delivered FIX is identical in amino acid sequence to the mature recombinant FIX used for systemic delivery, but lacks glycosylation and γ-carboxylation (and therefore does not have coagulation activity and cannot restore hemostasis). Previously, we also achieved tolerance using a CTB-FIX fusion without a furin cleavage site despite a 20-fold lower expression level.8 This result further supports the importance of antigen processing and presentation leading to the immune modulatory effect on the systemic response. However, we cannot formally exclude a contribution of systemic distribution of the orally delivered antigen to other sites such as the liver in the tolerance mechanism.
The tolerance regimen induced 2 types of Treg that suppressed anti-FIX formation. Induction of and sustained suppression by CD4+CD25+FoxP3+ Treg is a critical feature of several diverse experimental immune tolerance protocols for FIX.11,12,21 Antigen-specific CD4+CD25+FoxP3+ Tregs are effective at low numbers, and therefore their induction may not be obvious from total frequencies (Figure 3A) but can be demonstrated experimentally (Figures 1F and 5B). In contrast, overall frequencies of LAP+ among CD4+ T cells substantially increased (Figure 3B). Adoptive transfer showed conclusively that oral delivery of the transgenic plant material induced CD4+CD25−LAP+ Tregs (Figure 5D), representing first definitive evidence that this type of Treg can suppress antibody formation against coagulation factors. Furthermore, CD4+CD25−LAP+ Tregs upregulated TGF-β and IL-10 expression in response to FIX.
Multiple data sets (induction of LAP+ Treg, increase in CD103+ DCs, and production of IgA, a TGF-β–dependent class switch) indicate that TGF-β is a main mediator of the immune suppressive response. Interestingly, peripheral induction of CD4+CD25+FoxP3+ Treg is TGF-β–dependent, and these induced Tregs also express TGF-β. Hence, induction of 1 set of suppressive cells may upregulate another. IL-10 and TGF-β expression in Tregs may synergize in suppression. Ultimately, T helper cell responses and resulting inhibitor and IgE production are suppressed. Importantly, we were also able to reverse preexisting anaphylaxis and inhibitors. This result indicates that oral tolerance could be used in addition to or in place of current immune tolerance induction protocols, especially because the complication of anaphylaxis is addressed. It will be of interest to investigate whether there may be differences in the DC and Treg responses between naive and preimmune animals that receive the oral regimen.
Although Tregs of IL-10 deficient mice induced by hepatic FIX expression were able to suppress anti-FIX formation, IL-10 has a broadly important anti-inflammatory role in the intestine, where IL-10 production by CD4+CD25+FoxP3+ Treg is required.37,49 Inability to upregulate tolerogenic DC and T cells and ultimately failure to induce tolerance to FIX via the oral route in IL-10–deficient hemophilia B mice suggests that IL-10 is critical in the gut microenvironment. IL-10 expressing Tr1 cells in the LP may further enforce this immune regulation. It is also possible that other Treg subsets undergo expansion in the LP in our protocol, which warrants further study.50 In conclusion, this study provides the first detailed insights into the complex immune regulatory mechanism, summarized in supplemental Figure 14, that suppresses responses to therapeutic proteins upon oral delivery of plant cell–based antigen.
Authorship
Contribution: X.W., J.S., A.S., G.L.R., and B.E.H. performed experiments; X.W., G.L., K.W.L., C.T., H.D., and R.W.H. designed experiments; all authors participated in data analysis; X.W., G.L., C.T., H.D., and R.W.H. wrote the manuscript; and H.D. and R.W.H. conceived and supervised the study.
Conflict-of-interest disclosure: H.D. and R.W.H. have pending patent applications for plant-based oral tolerance for hemophilia. H.D. has awarded and pending patents on expression of human therapeutic proteins in chloroplasts. All other authors declare no competing financial interests.
Correspondence: Henry Daniell, University of Pennsylvania, 240 South 40th St, 547 Levy Building, Philadelphia PA 19104-6030; e-mail: hdaniell@upenn.edu; and Roland W. Herzog, University of Florida, Cancer and Genetics Research Center, 2033 Mowry Rd, Room 203, Gainesville, FL 32610; e-mail: rherzog@ufl.edu.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
The authors thank the flow cytometry core of the UF Health Shands Cancer Center for their assistance.
This work was supported by the National Institutes of Health, National Heart, Lung, and Blood Institute grant R01 HL107904 (H.D. and R.W.H.), grant R01 HL109442 (H.D., K.W.L., and R.W.H.), and Eunice Kennedy Shriver National Institute of Child Health and Human Development grant P01 HD078810 (C.T. and R.W.H).

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal