Key Points
Complete loss of KLF1 function is compatible with life but results in severe nonspherocytic hemolytic anemia and kernicterus.
Human KLF1 regulates most aspects of red cell biology.
Abstract
We describe a case of severe neonatal anemia with kernicterus caused by compound heterozygosity for null mutations in KLF1, each inherited from asymptomatic parents. One of the mutations is novel. This is the first described case of a KLF1-null human. The phenotype of severe nonspherocytic hemolytic anemia, jaundice, hepatosplenomegaly, and marked erythroblastosis is more severe than that present in congenital dyserythropoietic anemia type IV as a result of dominant mutations in the second zinc-finger of KLF1. There was a very high level of HbF expression into childhood (>70%), consistent with a key role for KLF1 in human hemoglobin switching. We performed RNA-seq on circulating erythroblasts and found that human KLF1 acts like mouse Klf1 to coordinate expression of many genes required to build a red cell including those encoding globins, cytoskeletal components, AHSP, heme synthesis enzymes, cell-cycle regulators, and blood group antigens. We identify novel KLF1 target genes including KIF23 and KIF11 which are required for proper cytokinesis. We also identify new roles for KLF1 in autophagy, global transcriptional control, and RNA splicing. We suggest loss of KLF1 should be considered in otherwise unexplained cases of severe neonatal NSHA or hydrops fetalis.
Introduction
In the 20th century, hydrops fetalis was most commonly caused by α-thalassemia major1 or hemolytic disease of the newborn (HDN).2 The former is now rare in the first world because of efficient antenatal screening based on automated measurement of red blood cell size. HDN is also encountered rarely as a result of routine administration of anti-D immune globulin to RhD-negative mothers.2 Now, a cohort of hydrops fetalis cases of uncertain etiology exists. Congenital dyserythropoietic anemia (CDA) presents later with mild to moderate anemia, erythroblastosis, and abnormal red blood cell morphology. The subclassification of CDA into types I to IV is based on specific morphologic abnormalities (ie, multinucleated erythroblasts), and the Ham test.3-6 The molecular etiology of most CDA subtypes has been solved; type I is caused by mutations in codanin-1 (CDN1), type II by mutations in SEC23B, and type III by mutations in KIF23.7-9 Rare type IV CDA cases are caused by a dominant mutation in a DNA-binding amino acid within the second zinc finger of KLF1, E325K.10-12 These cases display nonspherocytic hemolytic anemia (NSHA) with erythroblastosis and persistence of embryonic globins (Hb-Portland) and HbF.11 Here we describe a case of unexpected severe neonatal anemia with hydrops fetalis as a result of compound heterozygosity for null alleles of KLF1: W30X and R319EfsX34. The proband was born with severe NSHA, hepatosplenomegaly, and jaundice, which was difficult to control. There was marked erythroblastosis with erythroid expansion within the bone marrow, and marked hereditary persistence of fetal hemoglobin (HPFH). The proband underwent allogeneic bone marrow transplantation at 6 years of age but developed cerebral palsy as a result of kernicterus.
Murine Klf1 is a thoroughly studied master regulator of erythroid gene expression and is essential for erythropoiesis.13-16 Many different heterozygous KLF1 loss of function (LoF) mutations have been described in humans.12,17-20 These cases are asymptomatic and not anemic, but they display abnormal expression of certain red cell antigens such as BCAM/Lutheran, which is occasionally discovered as an In(Lu) phenotype.18 Other red cell transmembrane proteins such as CD44 and AQP1 are expressed at reduced levels in KLF1 heterozygotes, and these can be used for fluorescence-activated cell sorting (FACS)-based diagnoses.21 Heterozygous LoF KLF1 mutations also result in mildly elevated HbF and HbA2 levels, consistent with critical role for KLF1 in hemoglobin switching,17,22-25 and elevated zinc protoporphyrin (ZPP), consistent with a defect in iron incorporation into heme. This concurs with mouse transcriptome and ChIP-seq studies.26
We performed RNA-seq on the proband to define the KLF1-dependent transcriptome in humans for the first time; 819 erythroid genes were poorly expressed compared with normal definitive erythroblasts. Many have been previously reported as murine Klf1 target genes.27-30 Others such as KIF23, KIF11, MBNL2, and CTBP2 have not been previously reported. We found that KLF1 regulates the cell cycle, iron procurement, cytokinesis, transcription, autophagy, and RNA splicing. We also confirmed previously reported roles for KLF1 in heme biosynthesis, cytoskeleton assembly, blood group antigen expression, and hemoglobin switching.16,17,27,28 Given the midgestation lethality of Klf1−/− mice,14 we were surprised to discover that a human null for KLF1 could survive until birth. We believe this relates to HPFH, which is not present in mice because regulation of the HBG1 and HBG2 genes is primate specific (see Discussion). Given the very high carrier rate for LoF mutations in certain regions of the world, many cases of hydrops fetalis or late fetal loss caused by KLF1 deficiency have likely been missed.20
Methods
This study was performed under Mater Health HREC approval No. 1938A. Hematology and biochemistry was performed by Queensland Pathology. Hemoglobin variant quantification was performed using the Bio-Rad CDM System. The Australian Red Cross Blood Service tested for red blood cell serotypes with a range of Lutheran antisera. FACS was performed on whole blood using a BD FACSCanto II analyzer with anti-CD44-PE (Becton Dickinson #555479) and anti-CD235 (GPA)-FITC (Dako #F0870) antibodies. Polymerase chain reaction was performed on peripheral blood DNA to amplify the 3 exons of KLF1 (primers listed in supplemental Table 1, available on the Blood Web site). Polymerase chain reaction products were sequenced according to Sanger by the Australian Genome Research Facility. RNA-seq was performed on the proband’s circulating erythroblasts and parental buffy coat. Comparisons were made, with published RNA-seq data representing normal erythroid differentiation. Further molecular methods including cloning and bioinformatics are provided in the supplemental Methods, available on the Blood website.
Results
Clinical features
We investigated a boy with severe neonatal anemia who was born in a small hospital in Northern Australia. Routine antenatal visits were uneventful and the maternal full blood examination was normal. The mother attended hospital in labor at 38 weeks gestation, and cesarean delivery was performed because of the neonate’s breech presentation. The birth weight was 3148 g and mild hydrops fetalis was noted. The APGAR score was 0 at 10 minutes, requiring resuscitation. Marked hepatomegaly (below the umbilicus) and splenomegaly were noted. At 6 hours of age, the Hb level was 86 g/L and the bilirubin level was 183 μmol/L, and the neonate had jaundice. The blood film showed severe erythroblastosis (3100 nucleated red blood cells per 100 white blood cells), reticulocytes, and fragmented red cells (Table 1). Spherocytes were sometimes noted, but the direct antiglobulin test (DAT) was negative. The mean corpuscular volume (MCV) (114 fL), platelet count (153 × 109/L), and white blood cell count (12.1 × 109/L) were normal (Table 1).
Hematologic parameters of proband and family
| . | . | . | . | . | . | . | . | . | Hemoglobin typing . | KLF1 allele . | |||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Case . | Age . | Hb (g/L) . | Hct (%) . | RCC (× 1012/L) . | MCV (fL) . | Retics (× 109/L) (NR <100) . | n-RBC/100 WBC . | Bilirubin (μmol/L) . | HbF (NR <1%) . | HbA2 (NR 2.3-3.3%) . | Other . | Allele 1 . | Allele 2 . |
| Proband | 6 h | 86 | 28 | 2.49 | 112 | ND | 3117 | 183 | 100% | <0.1 | * | W30X | R319Efs34X |
| 6 mo | 65 | 19 | 2.26 | 82 | 325 | 140 | 86 | 76% | <0.1 | 24%* | |||
| 12 mo | 74 | 22 | 2.76 | 89 | 185 | 44 | ND | 50% | 1.2% | HbA† | |||
| 4 y | 64 | 21 | 2.22 | 94 | 270 | 72 | ND | ND | ND | ND | |||
| Mother | 24 y | 120 | 38 | 4.38 | 86 | ND | 0 | 9 | 3.5 | 3.3 | HbA | WT | R319Efs34X |
| Father | 25 y | 164 | 48 | 5.57 | 86 | ND | 0 | ND | 2.4 | 3.0 | HbA | W30X | WT |
| . | . | . | . | . | . | . | . | . | Hemoglobin typing . | KLF1 allele . | |||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Case . | Age . | Hb (g/L) . | Hct (%) . | RCC (× 1012/L) . | MCV (fL) . | Retics (× 109/L) (NR <100) . | n-RBC/100 WBC . | Bilirubin (μmol/L) . | HbF (NR <1%) . | HbA2 (NR 2.3-3.3%) . | Other . | Allele 1 . | Allele 2 . |
| Proband | 6 h | 86 | 28 | 2.49 | 112 | ND | 3117 | 183 | 100% | <0.1 | * | W30X | R319Efs34X |
| 6 mo | 65 | 19 | 2.26 | 82 | 325 | 140 | 86 | 76% | <0.1 | 24%* | |||
| 12 mo | 74 | 22 | 2.76 | 89 | 185 | 44 | ND | 50% | 1.2% | HbA† | |||
| 4 y | 64 | 21 | 2.22 | 94 | 270 | 72 | ND | ND | ND | ND | |||
| Mother | 24 y | 120 | 38 | 4.38 | 86 | ND | 0 | 9 | 3.5 | 3.3 | HbA | WT | R319Efs34X |
| Father | 25 y | 164 | 48 | 5.57 | 86 | ND | 0 | ND | 2.4 | 3.0 | HbA | W30X | WT |
n-RBC, nucleated red blood cell; ND, not determined; NR, normalized ratio; WBC, white blood cell.
A very fast eluting band was detectable at all of these time points.
HbA was increased because of transfusion.
Despite frequent exchange transfusions from 6 hours and phototherapy, the bilirubin peaked at 550 μmol/L by 48 hours. Viral serology for T.O.R.C.H. and Parvovirus B19 infection was negative or consistent with passive maternal transfer. A Heinz body screen was negative. There were no dysmorphic features or evidence of congenital heart or renal disease. A bone marrow biopsy showed marked erythroid hyperplasia (90%), with dyserythropoiesis, normal megakaryocytes, and myeloid cell differentiation (Figure 1B). Cytogenetic examination was normal. There were few bi- or trinuclear erythroblasts, so a definitive diagnosis of CDA was not reached. A comprehensive red-cell enzyme screen (supplemental Table 5) was normal, but this was undertaken in the presence of transfused red blood cells, so is likely to overestimate the endogenous enzyme activities. The proband required transfusions every 4 to 6 weeks and had ongoing evidence of hemolysis with erythroblastosis (Table 1 and Figure 1A), an elevated unconjugated bilirubin (range 30-100 μmol/L), and lactate dehydrogenase (range 400-1400 U/L) (Table 1). If transfusion was withheld, the hemoglobin level would decrease to <70 g/L, requiring recommencement (Table 1).
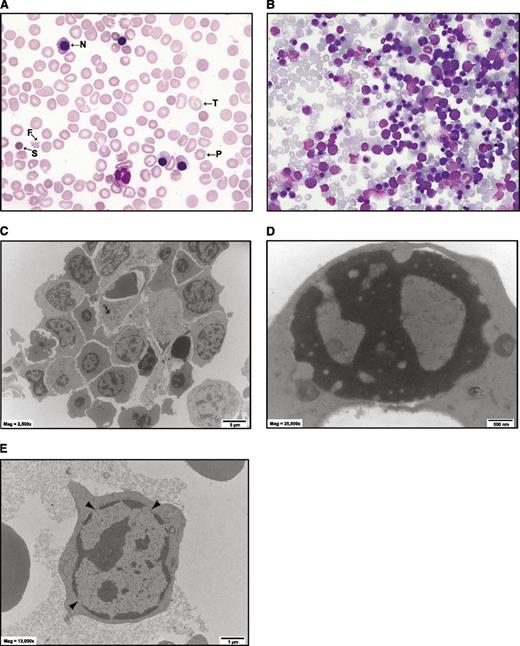
A case of atypical congenital dyserythropoietic anemia (CDA). (A) May-Grünwald-Giemsa (MGG)-stained blood film of patient at 4 years of age after blood transfusion. N, nucleated red blood cell; P, poikilocyte; F, fragmented cell; T, target cell; S, spherocyte. No pretransfusion film was available. (B) MGG-stained marrow biopsy at 1 week of age. More than 90% of the cells are erythroid. There are a few multinucleated cells that are characteristic of certain forms of CDA. (C-E) Electron microscopy of bone marrow. (C) Low-power view of a cluster of erythroid cells. The cell membranes are ruffled and there is some degree of dyserythropoiesis, but hyperploidy is not marked. (D) An erythroblast with a “Swiss cheese” pattern of nuclear chromatin. (E) An erythroblast with prominent gaps in the nuclear membrane.
A case of atypical congenital dyserythropoietic anemia (CDA). (A) May-Grünwald-Giemsa (MGG)-stained blood film of patient at 4 years of age after blood transfusion. N, nucleated red blood cell; P, poikilocyte; F, fragmented cell; T, target cell; S, spherocyte. No pretransfusion film was available. (B) MGG-stained marrow biopsy at 1 week of age. More than 90% of the cells are erythroid. There are a few multinucleated cells that are characteristic of certain forms of CDA. (C-E) Electron microscopy of bone marrow. (C) Low-power view of a cluster of erythroid cells. The cell membranes are ruffled and there is some degree of dyserythropoiesis, but hyperploidy is not marked. (D) An erythroblast with a “Swiss cheese” pattern of nuclear chromatin. (E) An erythroblast with prominent gaps in the nuclear membrane.
Clinical features suggesting CDA
The level of HbF remained very high (>70%) beyond 12 months (Table 1), with no evidence for common deletions or point mutations reported to cause HPFH (see Methods). HbA2 was below normal and an unidentifiable band with fast elution times was present. In view of the RNA-seq data (Table 1) and literature,11,19 this is likely to be Hb-Portland. By 12 months of age, the diagnosis of atypical CDA was considered. Electronmicroscopy studies on bone marrow showed mildly dysmorphic features but not those typical of CDA I, II, or III.31-33 In particular, there were few binucleate or multinucleate forms, no nuclear bridging, and few double membranes (Figure 1C). Electronmicroscopy features of thalassemia such as ferritin deposits were not prominent. Cell membranes were ruffled as previously reported for murine Klf1−/− erythroid cells.27,28 There were occasional erythroblasts that showed a “Swiss cheese” pattern of heterochromatin, as seen with CDA I (Figure 1D), and many showed wide nuclear pores as described in CDA IV (Figure 1E, arrowheads),11,33 but these were not considered diagnostic. From 12 months of age, clinical features of cerebral palsy became apparent and this was attributed to kernicterus. At 6 years of age, a successful unrelated allogeneic bone marrow transplant was undertaken.
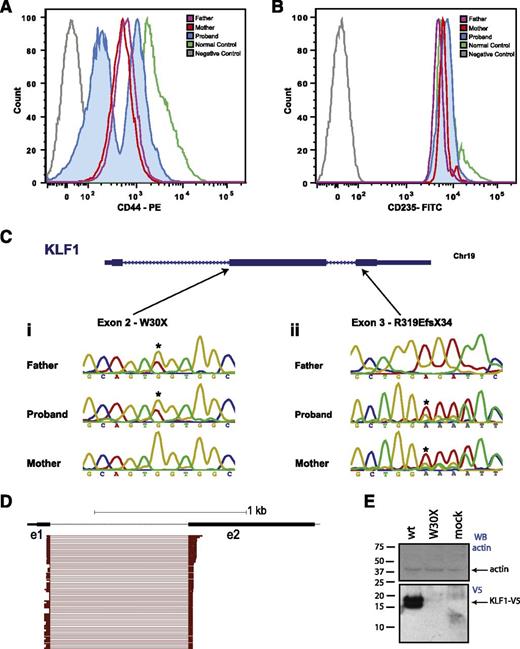
The proband was the only male child from unrelated parents. Both the mother and father had mildly elevated HbF, and the mother had elevated HbA2 (Table 1). FACS was performed on circulating red blood cells. Both parents displayed reduced expression of CD44, whereas CD235 (GPA) expression was normal (Figure 2A-B). The proband had 2 distinct red blood cell populations; one with normal levels of CD44 (transfused cells) and one with very low levels (endogenous cells) (Figure 2A). Both parents also had an In(Lu) serologic phenotype.
Inherited compound heterozygous mutations in KLF1. (A) FACS histogram overlay for CD44 expression on red blood cells for the mother, father, and proband compared with an isotype (negative) and a normal (positive) control. There is reduced expression of CD44 in both parents and 2 populations of CD44 red blood cells in the proband: CD44 high and CD44 absent. The CD44 high cells are transfused. (B) FACS histogram for CD235 (GPA) expression. There are no differences in CD235 surface expression among the normal control, the parents, and the proband. (C) Sanger sequencing traces for the parents and proband for the 2 KLF1 gene positions indicated: (i) at the start of exon 2, and (ii) the middle of exon 3. Asterisks indicate sequence variations that lead to W30X or R319EfX34 coding changes. (D) Subsampling of RNA-seq reads from the proband, filtered for those with a mapq score of ≥20 and map location to >1 exon. From a total of >100 e1-e2 splice junction tags, there were no examples of alternative mutation-skipping splice junctions. (E) Western blot of COS7 cells transfected with expression vectors for wild-type and W30X mutant KLF1 cDNA fused to a V5 tag. The bottom (anti-V5) blot shows robust expression of 2 KLF1-V5 bands in the wild-type transduced cells and no protein in the W30X-mutant transduced cells. Lane 3 is a mock-transfected control. The top panel is an antiactin blot to confirm equal protein loading.
Inherited compound heterozygous mutations in KLF1. (A) FACS histogram overlay for CD44 expression on red blood cells for the mother, father, and proband compared with an isotype (negative) and a normal (positive) control. There is reduced expression of CD44 in both parents and 2 populations of CD44 red blood cells in the proband: CD44 high and CD44 absent. The CD44 high cells are transfused. (B) FACS histogram for CD235 (GPA) expression. There are no differences in CD235 surface expression among the normal control, the parents, and the proband. (C) Sanger sequencing traces for the parents and proband for the 2 KLF1 gene positions indicated: (i) at the start of exon 2, and (ii) the middle of exon 3. Asterisks indicate sequence variations that lead to W30X or R319EfX34 coding changes. (D) Subsampling of RNA-seq reads from the proband, filtered for those with a mapq score of ≥20 and map location to >1 exon. From a total of >100 e1-e2 splice junction tags, there were no examples of alternative mutation-skipping splice junctions. (E) Western blot of COS7 cells transfected with expression vectors for wild-type and W30X mutant KLF1 cDNA fused to a V5 tag. The bottom (anti-V5) blot shows robust expression of 2 KLF1-V5 bands in the wild-type transduced cells and no protein in the W30X-mutant transduced cells. Lane 3 is a mock-transfected control. The top panel is an antiactin blot to confirm equal protein loading.
Compound heterozygosity for KLF1 mutations
KLF1 exon resequencing detected a frameshift mutation in maternal DNA, which results in a truncated version of KLF1 that cannot bind DNA (R319Efs34X) (Figure 2C). This is known to cause mild HPFH and In(Lu).34 Surprisingly, the father had a novel KLF1 mutation at the start of exon 2, leading to a predicted Tryptophan (W) to Amber (X) mutation at amino acid 30 (Figure 2C). Initially, we suspected this mutation might be skipped by an “in-frame” alternative splicing event, such that a variant KLF1 protein could have been generated. We speculated that this may be why the child was alive at birth, whereas Klf1−/− mice die in utero at midgestation.13,35 To address this possibility and to determine the function of KLF1 in human erythropoiesis, we performed RNA-seq on peripheral circulating blood cells of the proband and his parents, and compared this with published RNA-seq datasets (see Methods).36 RNA-seq confirmed compound heterozygosity for LoF mutations in KLF1. We found no evidence for alternative splicing to skip the W30X mutation (Figure 2D). The maternal KLF1 allele was represented at only ∼15% of the total KLF1 RNA, suggesting the maternal frameshift mutation leads to some degree of nonsense-mediated decay.
We also considered the possibility that the paternal allele might be translated from an alternative downstream methionine because Met-39 lies just distal to the mutation in a strong Kozac context. There were no erythroblasts available from the patient after bone marrow transplant to examine KLF1 protein levels, so we cloned the mutant (W30X) and wild-type alleles in frame with a V5 tag for expression in COS7 cells (see Methods). There was robust expression of the wild-type KLF1 protein of the expected size (∼15 kD) (Figure 2E), but no expression of the predicted shorter 10-kD protein derived from Met-39 of the W30X allele. Thus no functional protein expression is possible from this allele.
The KLF1-dependent erythroid transcriptome
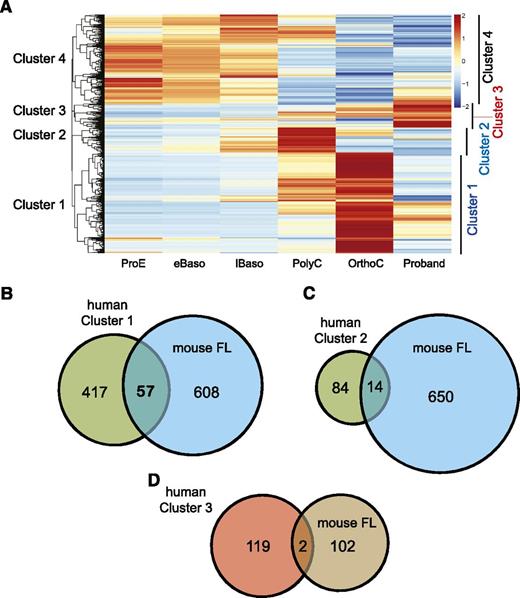
We mapped ∼20 million reads from each sample to hg19, and calculated an FPKM for all genes. Hierarchical clustering revealed 4 relatively distinct clusters of genes (Figure 3A). From these, we considered those genes with differential expression of >1.5 in either direction and ranked these (see supplemental Tables 2-4 for complete gene lists). The largest cluster of 472 differentially expressed genes (DEGs) (Figure 3A, Cluster 1) showed a pattern of upregulation during normal terminal differentiation but weak expression in the proband. The second cluster of 98 DEGs displayed a peak of expression in PolyC, then downregulation in OrthoC and low expression in the proband. We found a strong overlap between the genes in Clusters 1 and 2 with Klf1-dependent genes in the mouse (Figure 3B-C).29 We also found a KLF1 ChIP-seq peak within the promoter, introns, or within 20 kb of the transcriptional start site for many genes in Clusters 1 and 2.37 In some cases, we confirmed Klf1 occupancy at an equivalent position within the murine gene homolog.26 Thus, it is likely that many of the genes in Clusters 1 and 2 are direct human KLF1 target genes.
KLF1 differentially expressed genes. (A) Hierarchical clustering of RNA samples from ex vivo cultured primary erythroid cultures and the proband (column 6), color coded by expression level. Cluster 1 contains genes that are upregulated during normal terminal erythroid differentiation (OrthoC), but not in the proband. Cluster 2 contains genes, which are normally upregulated at the polychromatic erythroblast stage (PolyC), then down regulated in OrthoC, but not expressed in the proband. Cluster 3 contains genes that are more highly expressed in the proband than any of the stages of normal erythroid differentiation. Cluster 4 contains a list of genes that are highly expressed in normal proerythroblasts (ProE), then downregulated upon normal terminal differentiation. (B) Venn diagram of overlap of differentially expressed genes from Cluster 1 and from mouse Klf1-dependent genes as determined in reference 29 (ie, those genes with significantly less expression in fetal liver from Klf1−/− mice vs Klf1+/+ litter mates). (C) Overlap of differentially expressed genes from Cluster 2 with mouse Klf1 differentially expressed genes. (D) Overlap of differentially expressed genes from Cluster 3 with those genes upregulated in Klf1−/− mouse fetal liver.
KLF1 differentially expressed genes. (A) Hierarchical clustering of RNA samples from ex vivo cultured primary erythroid cultures and the proband (column 6), color coded by expression level. Cluster 1 contains genes that are upregulated during normal terminal erythroid differentiation (OrthoC), but not in the proband. Cluster 2 contains genes, which are normally upregulated at the polychromatic erythroblast stage (PolyC), then down regulated in OrthoC, but not expressed in the proband. Cluster 3 contains genes that are more highly expressed in the proband than any of the stages of normal erythroid differentiation. Cluster 4 contains a list of genes that are highly expressed in normal proerythroblasts (ProE), then downregulated upon normal terminal differentiation. (B) Venn diagram of overlap of differentially expressed genes from Cluster 1 and from mouse Klf1-dependent genes as determined in reference 29 (ie, those genes with significantly less expression in fetal liver from Klf1−/− mice vs Klf1+/+ litter mates). (C) Overlap of differentially expressed genes from Cluster 2 with mouse Klf1 differentially expressed genes. (D) Overlap of differentially expressed genes from Cluster 3 with those genes upregulated in Klf1−/− mouse fetal liver.
KLF1 and transcriptional repression
There is debate about whether KLF1 might act as a transcriptional repressor in some contexts.38,39 Cluster 3 contains 121 genes that were upregulated in the proband (Figure 3D and supplemental Table 3). These could be either genes that are normally repressed by KLF1 or genes artificially placed in this cluster by the normalization process (see Methods). However, only 2 genes in this list were also upregulated in Klf1−/− fetal liver. Thus, we conclude that most of these genes are not direct KLF1 target genes (ie, KLF1 rarely acts as a transcriptional repressor in vivo). An exception is carbonic anhydrase 1 (CA1) (Table 2), which is also upregulated in Klf1−/− fetal liver.28,29 There is a KLF1 ChIP-seq peak in the CA1 promoter (supplemental Figure 1A), so it might be a direct target. This analysis does not exclude a role for KLF1 in gene repression during megakaryopoiesis.39
Curated list of KLF1-dependent genes
| . | . | Absolute expression level (FPKM) . | P/O ratio . | Refs‡ . | |||||
|---|---|---|---|---|---|---|---|---|---|
| Gene symbol . | Alternate name or comment/function . | ProE . | eBaso . | lBaso . | Poly . | Ortho . | Proband . | ||
| Category 1: Transmembrane proteins/red blood cell antigens* | |||||||||
| LRP11 | Wnt coreceptor | 18.5 | 14.5 | 9.48 | 6.66 | 48.82 | 0.31 | 0.006 | |
| SLC6A19 | AA transporter | 0.10 | 0.48 | 3.59 | 24.15 | 32.97 | 0.30 | 0.009 | |
| SLC2A4 | Glut4 | 4.93 | 4.57 | 5.88 | 33.46 | 82.20 | 1.29 | 0.016 | 47 |
| ART4 | Dombrock Ag | 2.94 | 6.94 | 16.93 | 46.54 | 59.98 | 1.09 | 0.018 | 78 |
| GYPA | Glycophorin A | 78.7 | 136 | 274.6 | 719.7 | 663.8 | 8.01 | 0.012 | |
| SLC22A23 | Cation transporter | 4.83 | 6.21 | 10.10 | 66.95 | 195.4 | 3.20 | 0.016 | |
| SLC6A9 | Glycine transporter | 7.57 | 28.6 | 91.76 | 378.2 | 439.3 | 11.59 | 0.026 | |
| TMEM56 | Unknown function | 41.1 | 49.3 | 66.84 | 77.32 | 93.89 | 2.49 | 0.027 | |
| GYPE | Glycophorin E | 23.1 | 41.7 | 84.65 | 213.9 | 203.1 | 6.43 | 0.032 | |
| SLC16A1 | Lactate transporter | 14.1 | 9.97 | 4.99 | 34.24 | 245.3 | 8.40 | 0.034 | |
| SLC7A5 | AA transporter | 41.8 | 77.4 | 155.6 | 590.0 | 1396 | 48.73 | 0.035 | |
| CLCN3 | Chloride channel | 16.3 | 24.3 | 49.19 | 188.1 | 342.2 | 12.07 | 0.035 | |
| ERMAP | Scianna Ag | 39.6 | 56.1 | 98.29 | 277.4 | 681.2 | 33.42 | 0.049 | 79 |
| DARC | Duffy Ag | 3.84 | 5.75 | 14.06 | 50.03 | 52.14 | 2.64 | 0.051 | 29 |
| SLC14A1 | Kidd Ag | 0.57 | 1.33 | 5.77 | 46.87 | 179.7 | 10.19 | 0.057 | |
| XK | X-linked Kx blood group | 35.1 | 36.5 | 48.38 | 61.53 | 103.5 | 6.90 | 0.067 | |
| RHD | Major blood group | 2.00 | 9.81 | 35.09 | 86.75 | 103.3 | 7.16 | 0.069 | |
| SLC30A1 | Zinc transporter | 7.58 | 13.1 | 24.41 | 84.92 | 214.3 | 17.13 | 0.080 | |
| LRRC8A | Swell1– water channel | 4.50 | 5.76 | 8.83 | 61.02 | 201.8 | 24.12 | 0.120 | 50 |
| ICAM4 | Landsteiner-Wiener Ag | 42.5 | 49.2 | 78.35 | 82.47 | 68.9 | 10.94 | 0.159 | 49 |
| RHCE | Rh-associated | 2.75 | 12.9 | 49.61 | 121.0 | 109.4 | 30.41 | 0.278 | |
| KEL | Kell | 31.1 | 47.5 | 72.01 | 101.9 | 124.9 | 36.02 | 0.288 | 29 |
| RHAG† | Membrane complex | 181 | 207 | 316.8 | 687 | 393.9 | 23.67 | 0.03 | 29 |
| AQP1 | Water channel | 31.4 | 53.5 | 90.45 | 104 | 28.69 | 4.54 | 0.04 | 11 |
| Category 2: Cell division and cytokinesis | |||||||||
| TRIB3 | Tribbles 3 | 0.57 | 7.54 | 43.0 | 431.5 | 508.8 | 7.53 | 0.015 | |
| CEP76 | Centrosome | 4.21 | 5.05 | 7.09 | 17.55 | 51.84 | 1.26 | 0.024 | |
| KLC3 | Kinesin light-chain 3 | 0.65 | 0.51 | 0.43 | 10.37 | 62.50 | 2.53 | 0.040 | |
| STRADB | STE20-related kinase ad | 44 | 43.83 | 60.1 | 409.9 | 935.1 | 48.98 | 0.052 | |
| KLHL21 | Bric-c-brac family | 10.4 | 9.73 | 8.14 | 20.97 | 393.3 | 20.90 | 0.053 | 80 |
| E2F2 | S-phase regulation | 3.20 | 16.37 | 57.4 | 207.4 | 478.6 | 60.12 | 0.126 | 45,51 |
| SERTAD1 | Cdk4 inhibitor | 1.02 | 0.95 | 1.00 | 7.52 | 136.3 | 19.63 | 0.144 | |
| CCNA2 | Cyclin A | 57.3 | 79.70 | 136 | 168 | 49.19 | 4.08 | 0.02 | 81 |
| KIF11† | Eg5–mitosis regulation | 17.4 | 26.18 | 50.8 | 63.29 | 19.89 | 2.95 | 0.05 | |
| SKA1† | Spindle and kinetocore | 11.3 | 16.51 | 29.8 | 38.99 | 6.50 | 2.06 | 0.05 | |
| KIF23† | Mutated in CDA type III | 11.2 | 18.97 | 40.6 | 53.24 | 10.62 | 4.15 | 0.08 | 9 |
| CDKN2C† | p18 | 9.60 | 15.77 | 36.4 | 79.99 | 32.65 | 18.12 | 0.23 | 52 |
| Category 3: Cytoskeleton | |||||||||
| TBCEL | Tubulin folding | 2.64 | 2.35 | 2.87 | 62.41 | 466.4 | 19.80 | 0.042 | |
| ANKRD9 | Ank Repeat | 3.89 | 6.58 | 12.4 | 85.62 | 471.7 | 22.74 | 0.048 | |
| NEK7 | NIMA kinase | 5.75 | 6.95 | 10.6 | 31.37 | 63.20 | 3.93 | 0.062 | 57,82 |
| ANK1 | Ankyrin 1 | 93.4 | 118.1 | 174 | 450.6 | 824.0 | 66.58 | 0.081 | 83 |
| SLC4A1 | Band 3 | 145 | 501.4 | 1390 | 3984 | 5925 | 695 | 0.117 | |
| KANK2 | KN motif and ankyrin | 12.7 | 13.05 | 10.9 | 26.52 | 80.56 | 15.02 | 0.186 | |
| SPTB | β-spectrin | 66.5 | 113.54 | 202 | 455.9 | 365.6 | 99.08 | 0.271 | |
| SPTA1† | α-spectrin | 55.4 | 75.10 | 133 | 431.1 | 287.2 | 68.75 | 0.16 | |
| Category 4: Heme, iron procurement, and globin | |||||||||
| ABCG2 | Heme transporter | 1.68 | 3.33 | 8.81 | 25.97 | 69.9 | 0.26 | 0.004 | |
| SLC40A1 | Ferroportin | 76.1 | 58.92 | 43.1 | 268.2 | 1103 | 8.19 | 0.007 | |
| ABCB6 | Heme transporter | 11.4 | 19.04 | 39.4 | 175.4 | 506.4 | 13.46 | 0.027 | |
| HBD | δ-globin | 313 | 373.5 | 589 | 1415 | 2597 | 85.7 | 0.033 | |
| TSPO2 | Benzodiazepine receptor | 3.25 | 7.83 | 32.2 | 128.4 | 162.8 | 8.75 | 0.054 | 84 |
| TFR2 | Transferrin receptor | 66.9 | 62.6 | 76.7 | 157.5 | 158.4 | 10.72 | 0.068 | |
| ABCB10 | ABC-me heme transport | 77.4 | 104 | 168 | 191.2 | 140.8 | 10.25 | 0.073 | 85 |
| BPGM | Controls 2,3 DPG level | 12.8 | 17.99 | 32.6 | 403.8 | 3106 | 396 | 0.128 | |
| FCH | Heme synthesis | 60.9 | 71.13 | 105 | 270 | 473.1 | 69.28 | 0.146 | |
| HMBS | Heme biosynthesis | 163 | 309.4 | 614 | 1402 | 2685 | 449 | 0.167 | |
| CPOX† | Heme synthesis | 81.7 | 110.4 | 189 | 381.4 | 217.8 | 9.80 | 0.03 | |
| HEMGN† | Hemogenin | 101 | 243.6 | 542 | 1139 | 545.3 | 69.76 | 0.06 | |
| Category 5: Cell signaling molecules or enzymes | |||||||||
| SRGAP2C | Slit-Robo GTPase-activating protein | 1.57 | 2.49 | 5.55 | 32.15 | 57.5 | 0.46 | 0.008 | |
| B3GNT8 | A glucosyl transferase | 0.10 | 0.12 | 0.35 | 8.13 | 236.1 | 3.92 | 0.017 | |
| FBXO30 | Fbxw7, ubiquitin ligase | 4.48 | 4.11 | 5.70 | 102.2 | 581.3 | 10.17 | 0.017 | 86 |
| SORD | Sorbitol dehydrogenase | 23 | 16.6 | 8.74 | 1.86 | 44.3 | 1.05 | 0.024 | |
| FHDC1 | FH2 domain–containing 1 | 4.89 | 13.4 | 36.12 | 189.5 | 532.2 | 13.43 | 0.025 | |
| MSMO1 | Mono-oxygenase | 19.1 | 15.2 | 14.00 | 49.47 | 225.1 | 5.84 | 0.026 | |
| MOB1B | MOB kinase | 13.6 | 17.7 | 29.44 | 152.6 | 358.4 | 9.50 | 0.027 | |
| HES6 | Hairy enhancer of split | 93.1 | 176 | 276.9 | 382.5 | 201.6 | 6.54 | 0.032 | |
| PLEK2 | Pleckstrin 2 | 1.38 | 2.63 | 4.21 | 122.1 | 482.4 | 16.65 | 0.035 | 87 |
| DYRK3 | Kinase in stress erythropoiesis | 16.1 | 14.8 | 13.14 | 41.77 | 235.8 | 11.85 | 0.050 | 63 |
| RAPGEF2 | Rap guanine nucleotide exchange | 8.90 | 9.73 | 13.01 | 97.57 | 415.2 | 37.22 | 0.090 | 88 |
| PIGQ | phosphatidylinositol biogenesis | 39.3 | 60.2 | 99.13 | 135.9 | 163.7 | 24.95 | 0.152 | |
| BMP2K | BMP2-inducible kinase | 27.3 | 30.6 | 41.93 | 139.6 | 261.9 | 13.99 | 0.053 | |
| EPOR | Erythropoietin receptor | 58.1 | 73.9 | 101.3 | 200.3 | 204.1 | 12.33 | 0.060 | 89,90 |
| RAP1GAP | RAP1-specific GAP | 7.96 | 14.9 | 44.70 | 469 | 444.6 | 32.15 | 0.072 | |
| MAST3 | Microtubule S/T kinase | 3.22 | 4.50 | 7.57 | 43.14 | 256.3 | 18.57 | 0.072 | |
| AIDA | Axin inhibitor | 34.1 | 35.4 | 43.33 | 92.56 | 83.54 | 6.19 | 0.074 | |
| PIK3R2 | PI3K regulatory unit 2 | 27.1 | 34.2 | 45.64 | 60.38 | 286.0 | 33.17 | 0.116 | 61 |
| PBK† | PDZ-containing kinase | 16.9 | 22.3 | 35.32 | 47.76 | 7.50 | 0.13 | 0.001 | |
| PKDCC† | Cytoplasmic kinase | 3.78 | 6.31 | 11.13 | 34.68 | 15.44 | 0.18 | 0.01 | |
| Category 6: Transcription factors and nuclear proteins | |||||||||
| NSUN3 | NOP2/Sun, RNA methyltransferase | 2.02 | 2.45 | 2.55 | 22.41 | 264.7 | 9.61 | 0.036 | |
| BEX1 | LMO2 interaction | 9.09 | 8.04 | 7.47 | 28.08 | 38.34 | 1.60 | 0.042 | 91 |
| AFF1 | pTEFb interaction, elongation | 12.2 | 14.3 | 20.88 | 88.14 | 303.3 | 13.74 | 0.045 | |
| TAL1 | SCL, essential erythroid transcription factor | 44.8 | 58.9 | 93.64 | 275.6 | 382.1 | 19.15 | 0.050 | 92,93 |
| SSSCA1 | Sjögren Ag | 32.3 | 35.3 | 32.51 | 27.88 | 108.9 | 6.54 | 0.060 | |
| CTBP2 | Corepressor–binds KLF3 | 21.3 | 22.8 | 28.26 | 48.47 | 109.9 | 6.99 | 0.064 | 59 |
| FOXO3 | Antiapoptosis transcription factor | 4.91 | 6.74 | 13.47 | 141.5 | 468.1 | 35.67 | 0.076 | 58 |
| USP12 | Coactivator of transcription | 10.7 | 11.6 | 17.04 | 95.48 | 279.2 | 22.11 | 0.079 | |
| MBNL2 | Muscleblind-2–splicing factor | 3.62 | 5.67 | 14.56 | 86.09 | 140.5 | 11.27 | 0.080 | 94 |
| TCEANC | TF IIS–basal transcription | 1.19 | 1.65 | 2.97 | 23.53 | 104.9 | 8.45 | 0.081 | |
| ELF3 | Ets family transcription factor | 0.00 | 0.01 | 0.01 | 2.11 | 53.09 | 4.70 | 0.088 | |
| SOX6 | Essential transcription factor for erythropoiesis | 1.17 | 4.00 | 13.58 | 36.75 | 31.83 | 3.63 | 0.114 | 75,76,95 |
| ARID3A | Regulates transcription | 4.84 | 4.64 | 6.12 | 62.87 | 159.3 | 19.08 | 0.120 | 96 |
| EGR1 | Krox-24/Zif268 transcription factor | 1.00 | 3.87 | 8.80 | 59.25 | 75.43 | 9.67 | 0.128 | |
| SF3B3 | Splicing factor | 24.6 | 25.1 | 20.67 | 16.88 | 84.92 | 17.76 | 0.209 | |
| KLF1 | Decreased by RNA decay | 204 | 244 | 338.8 | 813.5 | 1446 | 397 | 0.275 | |
| GATA1 | Erythroid transcription factor | 123 | 137 | 174.4 | 238.4 | 391.4 | 118 | 0.301 | |
| Category 7: Other classes | |||||||||
| TSKU | Tsukushi, Wnt, and BMP inhibitors | 3.35 | 4.22 | 6.09 | 19.76 | 31.25 | 0.09 | 0.003 | 97,98 |
| IGF2 | Insulin-like growth factor | 0.11 | 1.13 | 6.33 | 7.46 | 44.91 | 0.14 | 0.003 | |
| GDF15 | Stress erythropoiesis | 1.44 | 5.92 | 28.3 | 1071 | 2982 | 9.54 | 0.003 | 99 |
| NUDT4P1 | Nudix domain hydrolase | 30.9 | 52.8 | 124 | 753.4 | 2082 | 26.6 | 0.013 | |
| ARG2 | Arginase | 2.38 | 4.70 | 19.1 | 368.8 | 443.7 | 5.24 | 0.012 | |
| RSC1A1 | Overlaps DDI2 (see below) | 20.2 | 20.41 | 20.7 | 59.27 | 132.5 | 2.07 | 0.016 | |
| TUFT1 | Tuftelin 1 | 0.41 | 0.54 | 1.00 | 36.00 | 148.1 | 5.45 | 0.037 | |
| DDI2 | DNA-damage inducible 1 homolog 2 | 14.4 | 15.73 | 16.6 | 46.44 | 107.1 | 9.38 | 0.088 | |
| TRIM58 | GATA1 target gene | 20.5 | 19.88 | 21.9 | 277.1 | 2189 | 337 | 0.154 | 81 |
| FCHO2 | Endocytosis | 6.83 | 7.91 | 13.2 | 42.48 | 62.18 | 2.85 | 0.046 | |
| LNX2 | Ligand of numb | 3.94 | 3.43 | 3.74 | 19.76 | 82.31 | 4.10 | 0.050 | |
| TSPAN5 | Tetraspanin 5 | 11.1 | 14.93 | 26.3 | 132.3 | 521.7 | 34.0 | 0.065 | |
| DCUN1D1 | SSCRO–Neddylation E3 | 21.4 | 23.94 | 33.8 | 138.7 | 176.7 | 12.13 | 0.069 | |
| KIAA0232 | Unknown Fx | 2.76 | 3.48 | 5.72 | 59.64 | 241.6 | 19.86 | 0.082 | |
| ATG4D | Autophagy, cleaved by caspase 3 | 8.75 | 13.07 | 23.0 | 78.49 | 197.1 | 21.62 | 0.110 | 100,101 |
| AMBRA1 | Autophagy, interacts with ULK1 | 7.77 | 7.68 | 8.62 | 40.74 | 180.4 | 20.35 | 0.113 | 66 |
| BBC3 | BH3-only protein PUMA, BAK activator | 6.21 | 8.91 | 16.8 | 83.31 | 170.6 | 20.82 | 0.122 | 102 |
| ULK1 | ATG1–autophagy | 3.39 | 4.78 | 9.29 | 70.24 | 192.3 | 24.89 | 0.129 | 103 |
| STX2† | Syntaxin 2 | 10.7 | 15.51 | 26.3 | 59.98 | 35.38 | 2.64 | 0.04 | |
| . | . | Absolute expression level (FPKM) . | P/O ratio . | Refs‡ . | |||||
|---|---|---|---|---|---|---|---|---|---|
| Gene symbol . | Alternate name or comment/function . | ProE . | eBaso . | lBaso . | Poly . | Ortho . | Proband . | ||
| Category 1: Transmembrane proteins/red blood cell antigens* | |||||||||
| LRP11 | Wnt coreceptor | 18.5 | 14.5 | 9.48 | 6.66 | 48.82 | 0.31 | 0.006 | |
| SLC6A19 | AA transporter | 0.10 | 0.48 | 3.59 | 24.15 | 32.97 | 0.30 | 0.009 | |
| SLC2A4 | Glut4 | 4.93 | 4.57 | 5.88 | 33.46 | 82.20 | 1.29 | 0.016 | 47 |
| ART4 | Dombrock Ag | 2.94 | 6.94 | 16.93 | 46.54 | 59.98 | 1.09 | 0.018 | 78 |
| GYPA | Glycophorin A | 78.7 | 136 | 274.6 | 719.7 | 663.8 | 8.01 | 0.012 | |
| SLC22A23 | Cation transporter | 4.83 | 6.21 | 10.10 | 66.95 | 195.4 | 3.20 | 0.016 | |
| SLC6A9 | Glycine transporter | 7.57 | 28.6 | 91.76 | 378.2 | 439.3 | 11.59 | 0.026 | |
| TMEM56 | Unknown function | 41.1 | 49.3 | 66.84 | 77.32 | 93.89 | 2.49 | 0.027 | |
| GYPE | Glycophorin E | 23.1 | 41.7 | 84.65 | 213.9 | 203.1 | 6.43 | 0.032 | |
| SLC16A1 | Lactate transporter | 14.1 | 9.97 | 4.99 | 34.24 | 245.3 | 8.40 | 0.034 | |
| SLC7A5 | AA transporter | 41.8 | 77.4 | 155.6 | 590.0 | 1396 | 48.73 | 0.035 | |
| CLCN3 | Chloride channel | 16.3 | 24.3 | 49.19 | 188.1 | 342.2 | 12.07 | 0.035 | |
| ERMAP | Scianna Ag | 39.6 | 56.1 | 98.29 | 277.4 | 681.2 | 33.42 | 0.049 | 79 |
| DARC | Duffy Ag | 3.84 | 5.75 | 14.06 | 50.03 | 52.14 | 2.64 | 0.051 | 29 |
| SLC14A1 | Kidd Ag | 0.57 | 1.33 | 5.77 | 46.87 | 179.7 | 10.19 | 0.057 | |
| XK | X-linked Kx blood group | 35.1 | 36.5 | 48.38 | 61.53 | 103.5 | 6.90 | 0.067 | |
| RHD | Major blood group | 2.00 | 9.81 | 35.09 | 86.75 | 103.3 | 7.16 | 0.069 | |
| SLC30A1 | Zinc transporter | 7.58 | 13.1 | 24.41 | 84.92 | 214.3 | 17.13 | 0.080 | |
| LRRC8A | Swell1– water channel | 4.50 | 5.76 | 8.83 | 61.02 | 201.8 | 24.12 | 0.120 | 50 |
| ICAM4 | Landsteiner-Wiener Ag | 42.5 | 49.2 | 78.35 | 82.47 | 68.9 | 10.94 | 0.159 | 49 |
| RHCE | Rh-associated | 2.75 | 12.9 | 49.61 | 121.0 | 109.4 | 30.41 | 0.278 | |
| KEL | Kell | 31.1 | 47.5 | 72.01 | 101.9 | 124.9 | 36.02 | 0.288 | 29 |
| RHAG† | Membrane complex | 181 | 207 | 316.8 | 687 | 393.9 | 23.67 | 0.03 | 29 |
| AQP1 | Water channel | 31.4 | 53.5 | 90.45 | 104 | 28.69 | 4.54 | 0.04 | 11 |
| Category 2: Cell division and cytokinesis | |||||||||
| TRIB3 | Tribbles 3 | 0.57 | 7.54 | 43.0 | 431.5 | 508.8 | 7.53 | 0.015 | |
| CEP76 | Centrosome | 4.21 | 5.05 | 7.09 | 17.55 | 51.84 | 1.26 | 0.024 | |
| KLC3 | Kinesin light-chain 3 | 0.65 | 0.51 | 0.43 | 10.37 | 62.50 | 2.53 | 0.040 | |
| STRADB | STE20-related kinase ad | 44 | 43.83 | 60.1 | 409.9 | 935.1 | 48.98 | 0.052 | |
| KLHL21 | Bric-c-brac family | 10.4 | 9.73 | 8.14 | 20.97 | 393.3 | 20.90 | 0.053 | 80 |
| E2F2 | S-phase regulation | 3.20 | 16.37 | 57.4 | 207.4 | 478.6 | 60.12 | 0.126 | 45,51 |
| SERTAD1 | Cdk4 inhibitor | 1.02 | 0.95 | 1.00 | 7.52 | 136.3 | 19.63 | 0.144 | |
| CCNA2 | Cyclin A | 57.3 | 79.70 | 136 | 168 | 49.19 | 4.08 | 0.02 | 81 |
| KIF11† | Eg5–mitosis regulation | 17.4 | 26.18 | 50.8 | 63.29 | 19.89 | 2.95 | 0.05 | |
| SKA1† | Spindle and kinetocore | 11.3 | 16.51 | 29.8 | 38.99 | 6.50 | 2.06 | 0.05 | |
| KIF23† | Mutated in CDA type III | 11.2 | 18.97 | 40.6 | 53.24 | 10.62 | 4.15 | 0.08 | 9 |
| CDKN2C† | p18 | 9.60 | 15.77 | 36.4 | 79.99 | 32.65 | 18.12 | 0.23 | 52 |
| Category 3: Cytoskeleton | |||||||||
| TBCEL | Tubulin folding | 2.64 | 2.35 | 2.87 | 62.41 | 466.4 | 19.80 | 0.042 | |
| ANKRD9 | Ank Repeat | 3.89 | 6.58 | 12.4 | 85.62 | 471.7 | 22.74 | 0.048 | |
| NEK7 | NIMA kinase | 5.75 | 6.95 | 10.6 | 31.37 | 63.20 | 3.93 | 0.062 | 57,82 |
| ANK1 | Ankyrin 1 | 93.4 | 118.1 | 174 | 450.6 | 824.0 | 66.58 | 0.081 | 83 |
| SLC4A1 | Band 3 | 145 | 501.4 | 1390 | 3984 | 5925 | 695 | 0.117 | |
| KANK2 | KN motif and ankyrin | 12.7 | 13.05 | 10.9 | 26.52 | 80.56 | 15.02 | 0.186 | |
| SPTB | β-spectrin | 66.5 | 113.54 | 202 | 455.9 | 365.6 | 99.08 | 0.271 | |
| SPTA1† | α-spectrin | 55.4 | 75.10 | 133 | 431.1 | 287.2 | 68.75 | 0.16 | |
| Category 4: Heme, iron procurement, and globin | |||||||||
| ABCG2 | Heme transporter | 1.68 | 3.33 | 8.81 | 25.97 | 69.9 | 0.26 | 0.004 | |
| SLC40A1 | Ferroportin | 76.1 | 58.92 | 43.1 | 268.2 | 1103 | 8.19 | 0.007 | |
| ABCB6 | Heme transporter | 11.4 | 19.04 | 39.4 | 175.4 | 506.4 | 13.46 | 0.027 | |
| HBD | δ-globin | 313 | 373.5 | 589 | 1415 | 2597 | 85.7 | 0.033 | |
| TSPO2 | Benzodiazepine receptor | 3.25 | 7.83 | 32.2 | 128.4 | 162.8 | 8.75 | 0.054 | 84 |
| TFR2 | Transferrin receptor | 66.9 | 62.6 | 76.7 | 157.5 | 158.4 | 10.72 | 0.068 | |
| ABCB10 | ABC-me heme transport | 77.4 | 104 | 168 | 191.2 | 140.8 | 10.25 | 0.073 | 85 |
| BPGM | Controls 2,3 DPG level | 12.8 | 17.99 | 32.6 | 403.8 | 3106 | 396 | 0.128 | |
| FCH | Heme synthesis | 60.9 | 71.13 | 105 | 270 | 473.1 | 69.28 | 0.146 | |
| HMBS | Heme biosynthesis | 163 | 309.4 | 614 | 1402 | 2685 | 449 | 0.167 | |
| CPOX† | Heme synthesis | 81.7 | 110.4 | 189 | 381.4 | 217.8 | 9.80 | 0.03 | |
| HEMGN† | Hemogenin | 101 | 243.6 | 542 | 1139 | 545.3 | 69.76 | 0.06 | |
| Category 5: Cell signaling molecules or enzymes | |||||||||
| SRGAP2C | Slit-Robo GTPase-activating protein | 1.57 | 2.49 | 5.55 | 32.15 | 57.5 | 0.46 | 0.008 | |
| B3GNT8 | A glucosyl transferase | 0.10 | 0.12 | 0.35 | 8.13 | 236.1 | 3.92 | 0.017 | |
| FBXO30 | Fbxw7, ubiquitin ligase | 4.48 | 4.11 | 5.70 | 102.2 | 581.3 | 10.17 | 0.017 | 86 |
| SORD | Sorbitol dehydrogenase | 23 | 16.6 | 8.74 | 1.86 | 44.3 | 1.05 | 0.024 | |
| FHDC1 | FH2 domain–containing 1 | 4.89 | 13.4 | 36.12 | 189.5 | 532.2 | 13.43 | 0.025 | |
| MSMO1 | Mono-oxygenase | 19.1 | 15.2 | 14.00 | 49.47 | 225.1 | 5.84 | 0.026 | |
| MOB1B | MOB kinase | 13.6 | 17.7 | 29.44 | 152.6 | 358.4 | 9.50 | 0.027 | |
| HES6 | Hairy enhancer of split | 93.1 | 176 | 276.9 | 382.5 | 201.6 | 6.54 | 0.032 | |
| PLEK2 | Pleckstrin 2 | 1.38 | 2.63 | 4.21 | 122.1 | 482.4 | 16.65 | 0.035 | 87 |
| DYRK3 | Kinase in stress erythropoiesis | 16.1 | 14.8 | 13.14 | 41.77 | 235.8 | 11.85 | 0.050 | 63 |
| RAPGEF2 | Rap guanine nucleotide exchange | 8.90 | 9.73 | 13.01 | 97.57 | 415.2 | 37.22 | 0.090 | 88 |
| PIGQ | phosphatidylinositol biogenesis | 39.3 | 60.2 | 99.13 | 135.9 | 163.7 | 24.95 | 0.152 | |
| BMP2K | BMP2-inducible kinase | 27.3 | 30.6 | 41.93 | 139.6 | 261.9 | 13.99 | 0.053 | |
| EPOR | Erythropoietin receptor | 58.1 | 73.9 | 101.3 | 200.3 | 204.1 | 12.33 | 0.060 | 89,90 |
| RAP1GAP | RAP1-specific GAP | 7.96 | 14.9 | 44.70 | 469 | 444.6 | 32.15 | 0.072 | |
| MAST3 | Microtubule S/T kinase | 3.22 | 4.50 | 7.57 | 43.14 | 256.3 | 18.57 | 0.072 | |
| AIDA | Axin inhibitor | 34.1 | 35.4 | 43.33 | 92.56 | 83.54 | 6.19 | 0.074 | |
| PIK3R2 | PI3K regulatory unit 2 | 27.1 | 34.2 | 45.64 | 60.38 | 286.0 | 33.17 | 0.116 | 61 |
| PBK† | PDZ-containing kinase | 16.9 | 22.3 | 35.32 | 47.76 | 7.50 | 0.13 | 0.001 | |
| PKDCC† | Cytoplasmic kinase | 3.78 | 6.31 | 11.13 | 34.68 | 15.44 | 0.18 | 0.01 | |
| Category 6: Transcription factors and nuclear proteins | |||||||||
| NSUN3 | NOP2/Sun, RNA methyltransferase | 2.02 | 2.45 | 2.55 | 22.41 | 264.7 | 9.61 | 0.036 | |
| BEX1 | LMO2 interaction | 9.09 | 8.04 | 7.47 | 28.08 | 38.34 | 1.60 | 0.042 | 91 |
| AFF1 | pTEFb interaction, elongation | 12.2 | 14.3 | 20.88 | 88.14 | 303.3 | 13.74 | 0.045 | |
| TAL1 | SCL, essential erythroid transcription factor | 44.8 | 58.9 | 93.64 | 275.6 | 382.1 | 19.15 | 0.050 | 92,93 |
| SSSCA1 | Sjögren Ag | 32.3 | 35.3 | 32.51 | 27.88 | 108.9 | 6.54 | 0.060 | |
| CTBP2 | Corepressor–binds KLF3 | 21.3 | 22.8 | 28.26 | 48.47 | 109.9 | 6.99 | 0.064 | 59 |
| FOXO3 | Antiapoptosis transcription factor | 4.91 | 6.74 | 13.47 | 141.5 | 468.1 | 35.67 | 0.076 | 58 |
| USP12 | Coactivator of transcription | 10.7 | 11.6 | 17.04 | 95.48 | 279.2 | 22.11 | 0.079 | |
| MBNL2 | Muscleblind-2–splicing factor | 3.62 | 5.67 | 14.56 | 86.09 | 140.5 | 11.27 | 0.080 | 94 |
| TCEANC | TF IIS–basal transcription | 1.19 | 1.65 | 2.97 | 23.53 | 104.9 | 8.45 | 0.081 | |
| ELF3 | Ets family transcription factor | 0.00 | 0.01 | 0.01 | 2.11 | 53.09 | 4.70 | 0.088 | |
| SOX6 | Essential transcription factor for erythropoiesis | 1.17 | 4.00 | 13.58 | 36.75 | 31.83 | 3.63 | 0.114 | 75,76,95 |
| ARID3A | Regulates transcription | 4.84 | 4.64 | 6.12 | 62.87 | 159.3 | 19.08 | 0.120 | 96 |
| EGR1 | Krox-24/Zif268 transcription factor | 1.00 | 3.87 | 8.80 | 59.25 | 75.43 | 9.67 | 0.128 | |
| SF3B3 | Splicing factor | 24.6 | 25.1 | 20.67 | 16.88 | 84.92 | 17.76 | 0.209 | |
| KLF1 | Decreased by RNA decay | 204 | 244 | 338.8 | 813.5 | 1446 | 397 | 0.275 | |
| GATA1 | Erythroid transcription factor | 123 | 137 | 174.4 | 238.4 | 391.4 | 118 | 0.301 | |
| Category 7: Other classes | |||||||||
| TSKU | Tsukushi, Wnt, and BMP inhibitors | 3.35 | 4.22 | 6.09 | 19.76 | 31.25 | 0.09 | 0.003 | 97,98 |
| IGF2 | Insulin-like growth factor | 0.11 | 1.13 | 6.33 | 7.46 | 44.91 | 0.14 | 0.003 | |
| GDF15 | Stress erythropoiesis | 1.44 | 5.92 | 28.3 | 1071 | 2982 | 9.54 | 0.003 | 99 |
| NUDT4P1 | Nudix domain hydrolase | 30.9 | 52.8 | 124 | 753.4 | 2082 | 26.6 | 0.013 | |
| ARG2 | Arginase | 2.38 | 4.70 | 19.1 | 368.8 | 443.7 | 5.24 | 0.012 | |
| RSC1A1 | Overlaps DDI2 (see below) | 20.2 | 20.41 | 20.7 | 59.27 | 132.5 | 2.07 | 0.016 | |
| TUFT1 | Tuftelin 1 | 0.41 | 0.54 | 1.00 | 36.00 | 148.1 | 5.45 | 0.037 | |
| DDI2 | DNA-damage inducible 1 homolog 2 | 14.4 | 15.73 | 16.6 | 46.44 | 107.1 | 9.38 | 0.088 | |
| TRIM58 | GATA1 target gene | 20.5 | 19.88 | 21.9 | 277.1 | 2189 | 337 | 0.154 | 81 |
| FCHO2 | Endocytosis | 6.83 | 7.91 | 13.2 | 42.48 | 62.18 | 2.85 | 0.046 | |
| LNX2 | Ligand of numb | 3.94 | 3.43 | 3.74 | 19.76 | 82.31 | 4.10 | 0.050 | |
| TSPAN5 | Tetraspanin 5 | 11.1 | 14.93 | 26.3 | 132.3 | 521.7 | 34.0 | 0.065 | |
| DCUN1D1 | SSCRO–Neddylation E3 | 21.4 | 23.94 | 33.8 | 138.7 | 176.7 | 12.13 | 0.069 | |
| KIAA0232 | Unknown Fx | 2.76 | 3.48 | 5.72 | 59.64 | 241.6 | 19.86 | 0.082 | |
| ATG4D | Autophagy, cleaved by caspase 3 | 8.75 | 13.07 | 23.0 | 78.49 | 197.1 | 21.62 | 0.110 | 100,101 |
| AMBRA1 | Autophagy, interacts with ULK1 | 7.77 | 7.68 | 8.62 | 40.74 | 180.4 | 20.35 | 0.113 | 66 |
| BBC3 | BH3-only protein PUMA, BAK activator | 6.21 | 8.91 | 16.8 | 83.31 | 170.6 | 20.82 | 0.122 | 102 |
| ULK1 | ATG1–autophagy | 3.39 | 4.78 | 9.29 | 70.24 | 192.3 | 24.89 | 0.129 | 103 |
| STX2† | Syntaxin 2 | 10.7 | 15.51 | 26.3 | 59.98 | 35.38 | 2.64 | 0.04 | |
Selected curated sublists of genes (FPKM >30) derived from the full set of KLF1-differentially expressed genes (supplemental Tables 1-3), sorted according to function or subcellular localization and ranked by fold change in each group relative to orthochromatic normoblasts (from Cluster 1) or polychromatic normoblasts (from Cluster 2; also differentiated below in the second footnote). TF, transcription factor.
Categories based on gene ontology (GO) classification and manual curation.
Genes downregulated in the proband vs the polychromatic erythroblast (from Cluster 2).
Additional table references appear in the supplemental Data.
KLF1 regulates embryonic and fetal-to-adult hemoglobin switching
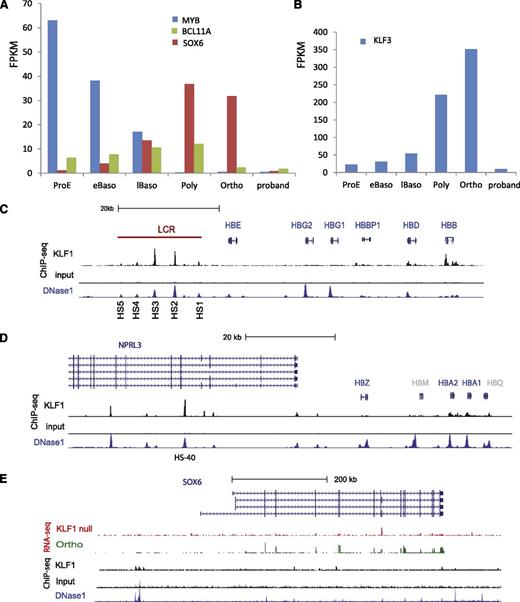
KLF1 binds the HBA1 and HBA2 gene promoters, and to HS-40 in the α-globin locus control region (LCR), which resides within the introns of the NPRL3 gene (Figure 4D). KLF1 also binds the β-globin gene promoter and DNase 1 HS sites in the β-globin LCR (Figure 4C). Equivalent sites are bound by Klf1 in the mouse.26 We found upregulation of the embryonic globin genes HBZ and HBE in the proband (supplemental Table 4), consistent with recent similar reports in patients with heterozygous LoF mutations in KLF1.11,19 However, we did not find evidence for KLF1 occupancy at the HBE, HBZ, HBG1, or HBG2 gene promoters in adult erythroid cells (Figure 4), consistent with results in the mouse,26 so we conclude that upregulation of embryonic and fetal globin genes is likely to be indirect. This could occur via KLF1-dependent globin gene–promoter competition for the LCR40 and/or via regulation of transcriptional repressors of the embryonic and fetal globin genes such as BCL11A, SOX6, MYB, KLF3, and others (reviewed in24 ). MYB regulates KLF1 and vice versa.41,42 KLF1 binds very strongly to an upstream (–80 kb) enhancer in the MYB locus, which is implicated in repression of fetal hemoglobin from GWAS studies.41,43 MYB is down regulated during normal erythroid differentiation (Figure 4A) to such a degree that we were not able to determine whether mRNA levels are further reduced in the proband (see Discussion). BCL11A was reduced in the proband compared with normal orthochromatic erythroblasts as it is in Klf1-null murine erythroid cells (Figure 4A).29 There is a complex enhancer within a large intron of BCL11A, which binds erythroid transcription factors including KLF1,44 so BCL11A is likely to be a direct KLF1 target gene. SOX6 and KLF3 are both normally upregulated during terminal erythropoiesis and markedly downregulated in late normoblasts of the proband (Figure 4 and Table 2) (see Discussion). We found a DNAse1 HS site ∼100 kb upstream of the SOX6 gene, which is occupied by KLF1 in erythroid cells (Figure 4E), so we propose that SOX6 is a direct target gene. In short, we find strong evidence for KLF1 regulation of transcriptional repressors of the embryonic and fetal globin genes. Combined impairment of their expression likely results in HPFH (See Discussion).
KLF1 regulates hemoglobin switching. (A-B) Absolute expression of MYB, BCL11A, SOX6, and KLF3 (in FPKM) during 5 stages of normal human erythroid cell differentiation from CD34+ cells compared with the proband. There is marked downregulation of SOX6 and KLF3 expression and moderate downregulation of BCL11A expression in the proband. (C) KLF1 occupies the human HBB gene promoter and 4 sites in the locus control region (LCR) that correspond to known DNase1 HS sites (K562 cells). KLF1 occupancy is greatest at HS2 and HS3. It does not occupy the HBE, HBG1, and HBG2 gene promoters in definitive erythroid cells. (D) KLF1 occupies the human HBA1 and HBA2 gene promoters and the HS sites at −40 kb (within the body of the NPRL3 gene) and further upstream. It does not bind the embryonically expressed HBAZ gene promoter in adult cells. HBM and HBQ are colored gray as they represent pseudogenes. (E) SOX6 expression is markedly reduced in KLF1-null erythroid cells (red RNA-seq track) compared with orthochromatic erythroblasts (green RNA-seq track). KLF1 occupies a site approximately 100 kb upstream of the most 5′ of the SOX6 exons (black track). This corresponds to a putative enhancer, as indicated by DNAse1 hypersensitivity in K562 cells (blue track). See Figure 5’s legend for explanation of the generation and coding of the custom tracks.
KLF1 regulates hemoglobin switching. (A-B) Absolute expression of MYB, BCL11A, SOX6, and KLF3 (in FPKM) during 5 stages of normal human erythroid cell differentiation from CD34+ cells compared with the proband. There is marked downregulation of SOX6 and KLF3 expression and moderate downregulation of BCL11A expression in the proband. (C) KLF1 occupies the human HBB gene promoter and 4 sites in the locus control region (LCR) that correspond to known DNase1 HS sites (K562 cells). KLF1 occupancy is greatest at HS2 and HS3. It does not occupy the HBE, HBG1, and HBG2 gene promoters in definitive erythroid cells. (D) KLF1 occupies the human HBA1 and HBA2 gene promoters and the HS sites at −40 kb (within the body of the NPRL3 gene) and further upstream. It does not bind the embryonically expressed HBAZ gene promoter in adult cells. HBM and HBQ are colored gray as they represent pseudogenes. (E) SOX6 expression is markedly reduced in KLF1-null erythroid cells (red RNA-seq track) compared with orthochromatic erythroblasts (green RNA-seq track). KLF1 occupies a site approximately 100 kb upstream of the most 5′ of the SOX6 exons (black track). This corresponds to a putative enhancer, as indicated by DNAse1 hypersensitivity in K562 cells (blue track). See Figure 5’s legend for explanation of the generation and coding of the custom tracks.
KLF1 coordinates expression of hundreds of genes that “build” a red blood cell
Based on GO terms and literature review, we found it convenient to group the DEGs into one of 7 categories: (1) transmembrane/blood group antigens, (2) genes involved in cell cycle and/or mitosis, (3) genes encoding cytoskeleton proteins, (4) genes involved in hemoglobin production, (5) cytoplasmic signaling molecules, (6) transcription factors and other nuclear proteins, and (7) genes that do not fit easily into one of the other 6 categories. For each of these categories, we displayed the top 10 to 20 DEGs in Table 2. The full lists are provided in supplemental Tables 2 and 3. Many are well-known murine Klf1 target genes,28,29,45 but some are novel. For each gene, we examined the UCSC Browser (hg19) after generation of wiggle tracks for RNA-seq data, ChIP-seq data,37 and DNase1 HS-seq data for K562 cells generated by ENCODE (see Methods).
KLF1 regulates genes that encode important blood groups
The KLF1-dependent gene set encodes many cell-surface proteins (Table 2, Category 1). There are 18 such genes expressed at <10% of wild-type levels in KLF1-null erythroid cells and many more expressed at 10% to 30%. These include well-known murine Klf1 target genes such as SLC2A4 (Glut4), SLC4A1 (Glut1), ERMAP (Scianna), DARC and KELL (Duffy and Kell), ICAM4 (Landsteiner-Wiener blood group), and AQP1.11,18,29,46 Glut1 and Glut4 are essential for normal red blood cell membrane integrity.47,48 ICAM4 is poorly expressed in Klf1−/− fetal liver and in patients with CDA IV.11,28,29 It is essential for interaction between developing erythroid cells and the central macrophage of erythroblastic islands, so it is likely to be critical for maturation of erythrocytes in vivo.49
Many Category 1 genes are occupied by KLF1 in primary human erythroid cells.37 For example, KLF1 binds to a site ∼500 bp upstream of the SLC2A4 gene promoter (supplemental Figure 1B), and the 2 alternative ERMAP gene promoters (supplemental Figure 1C). The equivalent genomic regions are bound by murine Klf1 in the fetal liver (supplemental Figure 2A-B).26 Furthermore, ChIP-seq peaks are associated with strong DNase1 hypersensitivity in K562 cells, suggesting that they have regulatory potential (supplemental Figure 1). A novel target of interest is the LRRC8A gene, which has recently been shown to encode an essential water channel, called SWELL1.50 KLF1 occupies the LRRC8A promoter and 2 sites in the first intron that are possible enhancers (supplemental Figure 1D). Together with loss of AQP1 (Table 2),11 loss of SWELL1 could contribute to osmotic fragility, which is a feature of KLF1-null red blood cells.
KLF1 regulates the cell cycle and mitosis
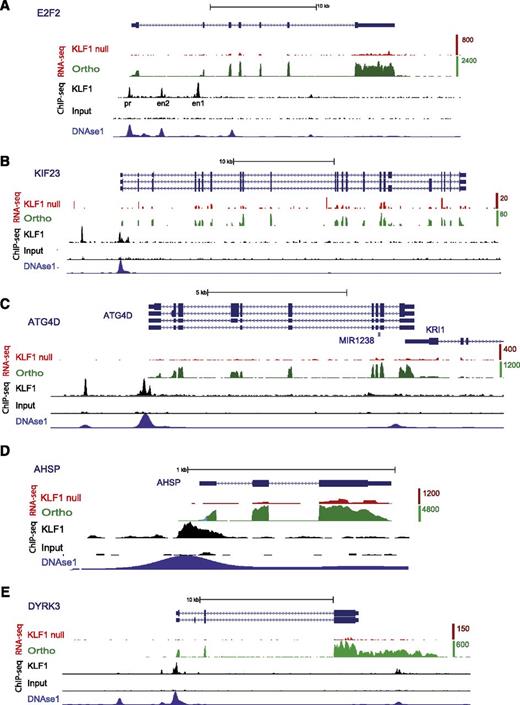
Many genes involved in cell-cycle control were downregulated in the proband (Category 2). We found 10 expressed at <15% of wild-type levels in KLF1-null cells (Table 2 and supplemental Table 2). E2f2, E2f4, p21-WAF1/CIP1, and p18-INK4c are all direct targets of murine Klf1, and cell-cycle perturbation is a major feature of Klf1−/− fetal liver cells.45,51-53 We found strong KLF1 ChIP-seq peaks within the promoter and first intron of E2F2 at positions previously reported in the mouse (Figure 5A).45,51 The site upstream of exon 2 has strong enhancer activity,51 so it is also likely to be a KLF1-depdendent enhancer for human E2F2. We found KLF1 binding at the promoter of the p18-INK4c (CDKN4C) gene (supplemental Figure 3A) at a site previously reported to bind murine Klf1 (supplemental Figure 2C).52
KLF1 regulates genes involved in cell-cycle control, cytokinesis, autophagy, globin synthesis, and cell signaling. Screen captures from the UCSC Genome Browser (hg19) with RefSeq transcripts for gene(s) at the top (blue) and the direction of transcription shown by arrowheads. All alternative transcripts from the RefSeq repository are included. Coding exons have an expanded vertical height compared with noncoding (5′ or 3′ untranslated region) per UCSC custom. The wiggle tracks for mapped and aligned RNA-seq tags are shown for the proband (KLF1 null, red) and orthochromatic erythroblasts (Ortho, green).36 Tags are highly enriched over exons as expected for polyA+ RNA-seq libraries. The top of the vertical scale for orthochromatic erythroblasts (tag counts) at each gene varies from 80 to 20 000, which reflects the broad range of gene expression detectable by RNA-seq (refer to supplemental Table 1 for FPKM values). The scale for the KLF1 RNA-seq track is 4 times lower to amplify its height to provide a “normalized” comparison with the green track (see Methods). Wiggle tracks for ChIP-seq data for KLF1 and input DNA (black) were uploaded from GEO.37 The wiggle track for DNase 1 HS-seq data from K562 cells generated by the ENCODE consortium (see Methods) was uploaded to show likely promoter and enhancer sites (bottom track, navy). KLF1 directly regulates expression of E2F2 (A), KIF23 (B), ATG4D (C), AHSP (D), and DYRK3 (E). The 5 genes are poorly expressed in the proband (red tracks) compared with orthochromatic erythroblasts (green tracks). ChIP-seq peaks at the promoter (pr) and 2 first intron enhancer sites (en1 and en2) are identical to previously described Klf1-occupied sites in the murine E2f2 gene.51 KLF1 binds the KIF23 promoter and a site ∼3 kb upstream, the ATG4D promoter and a site ∼1 kb upstream, the AHSP promoter, and the DYRK3 promoter and a site ∼3 kb upstream (black tracks). Most of these display erythroid DNAse1 hypersensitivity (blue track).
KLF1 regulates genes involved in cell-cycle control, cytokinesis, autophagy, globin synthesis, and cell signaling. Screen captures from the UCSC Genome Browser (hg19) with RefSeq transcripts for gene(s) at the top (blue) and the direction of transcription shown by arrowheads. All alternative transcripts from the RefSeq repository are included. Coding exons have an expanded vertical height compared with noncoding (5′ or 3′ untranslated region) per UCSC custom. The wiggle tracks for mapped and aligned RNA-seq tags are shown for the proband (KLF1 null, red) and orthochromatic erythroblasts (Ortho, green).36 Tags are highly enriched over exons as expected for polyA+ RNA-seq libraries. The top of the vertical scale for orthochromatic erythroblasts (tag counts) at each gene varies from 80 to 20 000, which reflects the broad range of gene expression detectable by RNA-seq (refer to supplemental Table 1 for FPKM values). The scale for the KLF1 RNA-seq track is 4 times lower to amplify its height to provide a “normalized” comparison with the green track (see Methods). Wiggle tracks for ChIP-seq data for KLF1 and input DNA (black) were uploaded from GEO.37 The wiggle track for DNase 1 HS-seq data from K562 cells generated by the ENCODE consortium (see Methods) was uploaded to show likely promoter and enhancer sites (bottom track, navy). KLF1 directly regulates expression of E2F2 (A), KIF23 (B), ATG4D (C), AHSP (D), and DYRK3 (E). The 5 genes are poorly expressed in the proband (red tracks) compared with orthochromatic erythroblasts (green tracks). ChIP-seq peaks at the promoter (pr) and 2 first intron enhancer sites (en1 and en2) are identical to previously described Klf1-occupied sites in the murine E2f2 gene.51 KLF1 binds the KIF23 promoter and a site ∼3 kb upstream, the ATG4D promoter and a site ∼1 kb upstream, the AHSP promoter, and the DYRK3 promoter and a site ∼3 kb upstream (black tracks). Most of these display erythroid DNAse1 hypersensitivity (blue track).
Some genes involved in mitosis or cytokinesis were downregulated in the proband (Table 2). Klf1-null erythroid cells are partly stalled in mitosis (in addition to the G1-S phase block), and there is a problem with cell division such that there are increased binucleated erythroid cells (ie, there is a cytokinesis defect,51 the hallmark of CDA). Interestingly, 2 kinesin genes, KIF11/Eg5 and KIF23, are expressed at <10% of wild-type levels in KLF1-null erythroid cells (Table 2), and KLF1 occupies the KIF23 promoter and a second upstream site (Figure 5B). KIF23 was recently found to be the gene mutated in families with CDA type III.9,54 Thus, there is a direct genetic link between KLF1 and CDA types IV and III. There are other novel KLF1 target genes that may be important for regulating mitosis including TRIB3, which controls cell division in response to erythropoietin.55
KLF1 regulates assembly of the cytoskeleton
The cell membrane is fragile in Klf1−/− erythroid cells, and hemolysis is a feature of human CDA IV and our case.11,27,28 We found 8 cytoskeletal genes expressed at <30% of wild-type levels in KLF1-null erythroid cells (Category 3). These include SPTB, SPTA1, SLC4A1, and ANK1, which is a thoroughly studied direct Klf1 target gene.27,56 KLF1 occupies many sites in the ANK1 locus (supplemental Figure 4A).56 A putative KLF1-occupied enhancer within one of the central introns is conserved (supplemental Figure 4A-B). There are additional cytoskeletal target genes that could contribute to the fragility of the red blood cell when missing56,57 (Table 2). Interestingly, Nek7−/− mice have nuclear hyperploidy, which is a feature of CDA.57
KLF1 regulates hemoglobin assembly
KLF1 regulates key erythroid transcription factors and cofactors.
We found 17 TFs or other nuclear proteins to be expressed at <30% of wild-type levels in KLF1-null erythroid cells (Category 6). Some of these have been noticed before as likely murine Klf1 target genes.29 SCL/TAL1, SOX6, FOXO3, and the Ets family member, ELF3, are downregulated in KLF1-null erythroid cells. Foxo3 is required in erythrocytes for protection from oxidative stress.58
The transcriptional corepressor, CtBP2, is expressed at just 6% of wild-type levels in KLF1-null erythroid cells, and its promoter is occupied by KLF1 (supplemental Figure 5B). CtBP2 binds a short N-terminal motif in certain KLF transcription factors such as KLF3, KLF8, and KLF12 to allow them to function as repressors59 (see Discussion). We found other KLF1-dependent nuclear proteins that are implicated in transcription factor interactions (BEX1 and USP12), transcription initiation (ATT1 and ARID3A), elongation (TCEANC), or alternative RNA splicing (MBNL2 and SF3B3). MBNL1 has been shown to play a critical role in alternative RNA splicing in erythroid cells,60 so MBNL2 is likely to do the same.
KLF1 regulates key cell-signaling molecules
We found that 18 genes associated with cell signaling were downregulated to <12% of wild-type levels in KLF1-null erythroid cells (Category 5). These include genes involved in EpoR signaling, enucleation,61,62 and stress erythropoiesis63 (Table 2). PLEK2 (Pleckstrin-2) and DYRK3 both have proven roles in terminal erythroid differentiation,63,64 and both are expressed at <10% of wild-type levels in the proband. There is KLF1 occupancy of the DYRK3 gene promoter (Figure 5E).
KLF1 regulates genes involved in autophagy
Autophagy is critical for removal of organelles, particularly mitochondria and nuclei during terminal erythroid differentiation.65 We found genes that encode critical effectors of autophagy to be KLF1-dependent (Table 2). These include ATG4D, ULK1/ATG1, and AMBRA1, a protein that interacts with ULK1.66 ATG4D and ULK1 have proven roles in mitochondrial clearance in reticulocytes.67,68 KLF1 binds the ATG4D promoter and a site ∼1 kb upstream of the transcriptional start site, which could be an enhancer (Figure 5C). Klf1 and Gata1 bind similarly to murine Atg4D (supplemental Figure 2E), so KLF1 is likely to regulate autophagy to generate mature red blood cells.
Discussion
We describe the first case of a KLF1-null human. The phenotype is perinatal NSHA with severe erythroblastosis and jaundice, leading to kernicterus. There were occasional binucleate cells but not as prominent as those found in CDA types I to III. It was a surprise to discover a KLF1-null human could survive to birth given the midgestation lethality of Klf1−/− mice,13,14 but we found no evidence for capacity to produce any KLF1 protein in the proband. Further cases will determine whether our case is typical or whether prenatal lethality is more common. The carrier frequency for loss-of-function KLF1 mutations is very high in some communities20,69 but not easy to detect by routine automated full blood analysis because the MCV is usually at the lower end of normal.20 Thus we suspect further cases of third-trimester fetal loss with hydrops fetalis may be caused by unrecognized cases of KLF1 deficiency. Antenatal diagnosis of KLF1 gene mutations by DNA resequencing could be offered to families at risk if the risk was appreciated. FACS for surface CD44 expression (possibly in combination with other cell-surface proteins from Table 2) might be a simple antenatal screening method to detect carriers or at-risk pregnancies.21
We found that KLF1 regulates many of the same pathways known to be regulated by Klf1 in the mouse, and those known to be required for terminal erythroid cell maturation. We found additional processes such as RNA splicing, cytokinesis, and autophagy to be KLF1-dependent. In fact, we suggest that most of the genes in Table 2 will play important roles in terminal red blood cell differentiation because they are KLF1-dependent. Although the ChIP-seq data are suggestive of direct regulation of many DEGs by KLF1, further detailed reporter assays are required to prove this gene by gene.
This case confirms that KLF1 plays an important role in globin gene-switching, both embryonic to adult, and fetal to adult. This is true for both the α-globin gene and the β-globin gene loci. KLF1 binds to the adult HBA1, HBA2, and HBB gene promoters and to key DNase1 HS sites in the α- and β-globin LCRs, but it does not bind the HBZ, HBE, HBG1, and HBG2 gene promoters in adult erythroid cells (supplemental Figure 2B-C). Thus we argue that KLF1 is not a direct repressor of embryonic and fetal globin genes, but acts indirectly via other TFs such as KLF3, BCL11A, and SOX6 and/or via competitive looping to the LCR70 to repress embryonic and fetal globin genes in adults.24
KLF3 is KLF1-dependent (Figure 4) because it is in the mouse.24 We found the transcriptional corepressor CtBP2 is also a direct KLF1 target gene (Table 2 and supplemental Figure 5). CtBP2 interacts with the N-terminus of KLF3 and related KLFs to provide a repressor function.71 Mice lacking both Klf3 and Klf8 fail to repress embryonic globin gene expression in vivo,72 so regulation of CtBP2 could be an alternative mechanism by which KLF1 indirectly regulates embryonic and fetal globin gene silencing (ie, via “enabling” the repression functions of KLF3 and KLF8). We also found that KLF1 regulates SOX6, which plays a key role in definitive erythropoiesis and embryonic globin gene silencing.73-75 Murine Sox6 interacts with Bcl11a and CtBP2.76,77 Thus it is therefore not surprising to find that KLF1 mutations, deletions, or siRNA knockdown experiments lead to a dramatic reactivation of fetal and embryonic globin genes.17,19,22,23,25 Given the absolute HPFH, this case will further inspire those interested in KLF1 as a drug target for small-molecule inhibitors to reactivate fetal globin in patients with sickle cell disease and other β-hemoglobinopathies. Genetic studies from China show that a 50% reduction in KLF1 levels leads to a marked clinical improvement in β-thalassemia major through increased expression of HbF,20 and this is likely to be the same for sickle cell disease. KLF1 knockdown to <50% of endogenous levels might provide additional benefit, but there must be some threshold below which unwanted defects in gene expression within erythroblasts would almost certainly be detrimental, as is seen in this case.
Sequencing data for this study has been deposited into the NCBI Gene Expression Omnibus under accession #GSE60514.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
FACS was performed by Noel Williams and David Gillis from Queensland Pathology.
This study was supported by National Health and Medical Research Council project APP1030143.
Authorship
Contribution: G.W.M., M.R.T., K.R.G., C.C.B., and N.M. performed experimental work for this publication; G.W.M., M.R.T., and A.C.P. prepared the figures and manuscript; B.W. was the primary clinical care provider; G.W.M., M.R.T., K.R.G., B.W., and A.C.P. contributed to the design of the project; and all authors read and approved the final manuscript.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Andrew Perkins, Mater Research Institute, The University of Queensland, Translational Research Institute, 37 Kent St, Woolloongabba, QLD, 4102, Australia; e-mail: andrew.perkins@mater.uq.edu.au.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal