Key Points
The N-terminal subunit of MSP1 binds to a specific polypeptide region of GPA during merozoite invasion of human RBCs.
The GPA–band 3 complex plays an essential role during malaria parasite invasion.
Abstract
Plasmodium falciparum invasion of human red blood cells (RBCs) is an intricate process requiring a number of distinct ligand-receptor interactions at the merozoite-erythrocyte interface. Merozoite surface protein 1 (MSP1), a highly abundant ligand coating the merozoite surface in all species of malaria parasites, is essential for RBC invasion and considered a leading candidate for inclusion in a multiple-subunit vaccine against malaria. Our previous studies identified an interaction between the carboxyl-terminus of MSP1 and RBC band 3. Here, by employing phage display technology, we report a novel interaction between the amino-terminus of MSP1 and RBC glycophorin A (GPA). Mapping of the binding domains established a direct interaction between malaria MSP1 and human GPA within a region of MSP1 known to potently inhibit P falciparum invasion of human RBCs. Furthermore, a genetically modified mouse model lacking the GPA– band 3 complex in RBCs is completely resistant to malaria infection in vivo. These findings suggest an essential role of the MSP1-GPA–band 3 complex during the initial adhesion phase of malaria parasite invasion of RBCs.
Introduction
Malaria remains one of the most common and deadly parasitic diseases in the world. The World Health Organization estimates that 5% to 9% of the global population is infected by malaria annually, resulting in over 700 000 deaths.1 Children younger than 5 years are most vulnerable to Plasmodium falciparum, the most lethal species among 4 malaria parasites that commonly infect humans. Malaria also takes an enormous economic toll by impacting the economies of most endemic countries, particularly in sub-Saharan Africa. Historically, vaccines have been one of the most effective means of controlling infectious diseases. Accumulating evidence suggests that a malaria vaccine can be developed2,3 ; however, most malaria vaccine candidate antigens have suffered limitations of low immunogenicity and efficacy. To date, no licensed vaccine exists for malaria despite the urgent global need.
Clinical manifestation and mortality in malaria is directly associated with the blood stage of the parasite life cycle. The invasion process consists of multiple molecular events during which red blood cell (RBC) membrane proteins and merozoite-coat proteins are engaged in specific receptor-ligand interactions to form unique invasion pathways.4,5 Continued interest in developing a multiantigen malaria vaccine has drawn much attention to parasite proteins localized on the surface and at the apical end of the merozoite as potential vaccine candidates.4 Merozoite surface protein 1 (MSP1) is a major ligand coating the surface of merozoites in all species of malaria parasites6 and is considered one of the leading candidates for inclusion in a multiple-subunit vaccine against malaria.7 People living in malaria-endemic areas carry MSP1-specific antibodies in their blood,8 and immunization of mice with Plasmodium yoelii MSP1 protects the animals from otherwise lethal P yoelii challenge.9 MSP1 undergoes extensive proteolytic processing either during schizogony or soon after the merozoites’ release from infected RBCs.10 Four major proteolytic fragments of P falciparum MSP1 include 83-kDa, 30-kDa, 38-kDa, and 42-kDa fragments, which remain associated as a noncovalent complex at the merozoite surface. The secondary proteolytic processing of the 42-kDa fragment results in the generation of 33-kDa and 19-kDa fragments. During the invagination phase of parasite entry, all MSP1 fragments are shed from the merozoite surface except the 19-kDa fragment, which remains attached to the merozoite surface through its glycosylphosphatidylinositol anchor, and eventually enters the RBC and modulates specific intracellular functions.11 Our previous studies demonstrated a biochemical and functional interaction between host RBC band 3 and P falciparum 42-kDa and 19-kDa fragments of MSP1.12,13 The obligatory role of MSP1 in malaria parasite invasion of RBCs14 suggests that other MSP1 segments may recognize cognate host receptors, thus serving as targets of an effective immune response against malaria.
In this study, using a P falciparum phage display library screen, we identified a novel region of MSP1 that directly binds to host RBC glycophorin A (GPA). Both in vitro and in vivo evidence support a functional role of the newly identified ligand-receptor interaction during merozoite invasion of RBCs. Together, our results suggest an essential role of the MSP1-GPA–band 3 complex during the malaria parasite invasion of RBCs.
Materials and methods
General methods, MSP1 constructs, and recombinant fusion proteins
All studies were Institutional Animal Care and Use Committee approved. P falciparum FCR3 strain was maintained in continuous culture according to standard conditions.15 The phage library screen against purified glycophorins was performed as described previously.16 We used a bacteriophage display library constructed from P falciparum complementary DNA and cloned into a T7Select10-3 OrientExpress vector. Phage particles were propagated in BLT5403 Escherichia coli strain to express the phage clones containing malaria proteins on the capsid surface for biopanning experiments. Multiple rounds of biopanning were performed on both intact human erythrocytes and purified immobilized glycophorins. Eluted phage particles were amplified in E coli BLT5403 strain, and the supernatant was used for biopanning. Specific phage clones were identified via polymerase chain reaction (PCR) amplification of individual plaques. The N-terminal fragment of MSP1 (MSP183A; 58-939 bp) was PCR amplified from P falciparum (FCR3) genomic DNA using the primers 5′-GCCGGATCCGTAACACATGAAAGTTATC-3′ (sense, BamHI) and 5′-GCCGTCGACAAGTAATTCCTTAATGTTT-3′ (antisense, SalI). The C-terminal fragment of MSP1 (MSP183B) (940-2007 bp) was amplified using the primers 5′-GCCGGATCCGATAAGATAAATGAAATT-3′ (sense, BamHI) and 5′-GCCGTCGACTTTTAATTTATCTACTTCT-3′ (antisense, SalI). PCR products were digested with BamHI and SalI and ligated in pET32a expression vector. The recombinant MSP183A and MSP183B were expressed in E coli BL21 (DE3) as fusions to the thioredoxin (TRX) and His-tag, and fusion proteins were affinity purified using S protein agarose beads. A 141-bp (631-771 bp encoding 47 amino acids) region of MSP183A (named here as MSP15kDa or MSP15) was amplified from MSP183A and cloned into pGEX-6P-2 using the primers 5′-GCCGGATCCCTTAAAAAACTTGTGTTC-3′ (sense, BamHI) and 5′-GCCGTCGACAATTGTTTTCTTACTTTC-3′ (antisense, SalI). A 309-bp region (103 amino acids) of MSP183A (named here as MSP112kDa or MSP112) was amplified from MSP183A and cloned into pET32a using primers 5′- GCGGATCCCTTAAAATTCGTGCAAATGAATTA-3′ (sense, BamHI) and 5′- GCGTCGACTAAAGTGTCAATACGTTTTTCTAA-3′ (antisense, SalI). Recombinant proteins were purified using ProBond Nickel-Chelating Resin (Life Technologies). Recombinant proteins TRX and MSP1 were dialyzed against 300 mM sodium chloride (NaCl), 20 mM N-2-hydroxyethylpiperazine-N′-2-ethanesulfonic acid pH 7.4, 0.5 mM EDTA, 5 mM magnesium chloride (MgCl2), 1 mM dithiothreitol (DTT), and 5% glycerol or RPMI 1640, whereas glutathione S-transferase (GST), GST-GPA* fusion protein (amino acids 31-72), and GST-GPAo fusion protein (amino acids 1-30) were dialyzed against 50 mM Tris-HCl pH 8.0, 300 mM NaCl, 0.5 mM DTT, and 1% glycerol. All proteins were sterile filtered prior to invasion assays.
Glycophorin and RBC binding assays
TRX-MSP183A, TRX-MSP183B, and TRX proteins were mixed with 7.5 μg of solubilized glycophorins, predominantly GPA (Sigma-Aldrich), in 600 μL of binding buffer (150 mM NaCl, 20 mM N-2-hydroxyethylpiperazine-N′-2-ethanesulfonic acid, 0.1% octylphenoxy poly(ethyleneoxy)ethanol (NP-40), 5 mM MgCl2, 10% glycerol, 1.0 mM DTT, and 0.5 mM EDTA pH 7.5), followed by incubation with 20 μL of packed S protein agarose beads (Novagen) overnight at 4°C with gentle mixing. Bound proteins were detected by immunoblotting. Both monoclonal and polyclonal antibodies against GPA were used.17 A polyclonal antibody against the cytoplasmic domain of glycophorin C (GPC)18 was used in the immunoblotting assays. The RBC binding assays were performed with 20 μL of fresh intact human erythrocytes in a buffer containing 50 mM Tris-HCl pH 8.0, 200 mM NaCl, 1.0 mM EDTA, 2.5 mM MgCl2, 2.0 mM DTT, and 1% glycerol. Binding was performed at 37°C for 2 hours, erythrocytes were washed 3 times in 1.0 mL of buffer, and bound proteins were eluted using 2.0 M NaCl. Binding assays using polypeptide regions of GPA were performed by immobilizing GST, GST-GPA*, and GST-GPAo on glutathione beads. MSP112 was incubated in 50 mM Tris-HCl pH 8.0, 200 mM NaCl, 2.5 mM MgCl2, 1.0 mM EDTA, 0.1% NP-40, 2.0 mM DTT, and 1.0 mg/mL of bovine serum albumin for 2 hours at room temperature. Beads were washed 3 times and eluted in sodium dodecyl sulfate sample buffer and analyzed by immunoblotting. TRX was used as a negative control.
RBC ghosts pull-down and invasion assays
MSP112 (TRX as control) was incubated with 40 μL of human RBC ghosts in 1% bovine serum albumin (RPMI 1640) for 1 hour at 4°C. Ghosts were centrifuged at 18 000g for 10 minutes at 4°C, and pellet was solubilized in modified radioimmunoprecipitation assay buffer (150 mM NaCl, 50 mM Tris-HCl pH 8.0, 1% NP-40, 7 mM 2-mercaptoethanol, 0.1 mM phenylmethylsulfonyl fluoride, and P8340 protease inhibitor cocktail from Sigma-Aldrich). Lysate was added to 25 μL of nickel beads for 2 hours at 4°C, and proteins bound to beads were eluted and analyzed by immunoblotting. Parasites used in the invasion assays were synchronized twice using 5% sorbitol treatment to isolate ring-stage parasites. Synchronized P falciparum FCR3 parasites at the schizont stage were seeded into a 96-well plate at 2% parasitemia (2% hematocrit) in 200 to 300 μL of total volume. Increasing amounts of recombinant protein in complete malaria media were added to infected erythrocytes, and TRX protein was used as a negative control. RBC invasion occurred under standard malaria culture conditions, and samples were analyzed after 20 to 24 hours postinvasion. It is noteworthy that the binding assays require a reducing agent (DTT) for proper MSP1 function and stability. However, when DTT is added to parasite culture, it often exerts toxic effects and significantly reduces overall parasitemia. This limitation can be controlled by using TRX and measuring relative parasitemia compared to the control. Thus, in the presence of DTT, MSP112 inhibited parasite invasion at high concentrations (10 μM) relative to TRX. RBC invasion was quantified using both microscopy and flow cytometry. For microscopy, at least 1000 RBCs were counted for each measurement. For flow cytometry, parasitemia was quantified using either propidium iodide staining (FACSCalibur)19 or Hoechst 33342 staining (BD LSR II flow cytometer).20 Invasion assays were performed in triplicate.
Infection of band 3 and protein 4.2 null mice by P yoelii 17XL
The generation and characterization of band 3 null mice has been described previously.21,22 Our band 3 null mouse model is unique because it selectively lacks erythroid band 3 but not its isoform in the kidney.21 Band 3 null mice display a secondary loss of GPA and protein 4.2 in the RBC membrane.22 Band 4.2 null mice were generously provided by Dr Luanne Peters at The Jackson Laboratory (Bar Harbor, Maine).23 Band 3 null and protein 4.2 null mice (2-3 months old) were challenged with the rodent P yoelii 17XL infection (blood stage), essentially as described previously.24 Wild-type C57BL/6 mice and age-matched heterozygous littermates were used as controls.
Results
Identification of P falciparum proteins binding to erythrocyte glycophorins
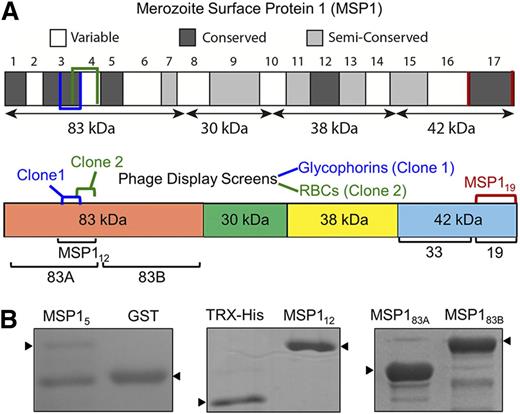
We used phage display technology to identify parasite invasion ligands interacting with glycophorins on the human RBC surface. Previously, we used the same approach to successfully identify a specific segment of P falciparum EBL-1 binding to RBC glycophorin B (GPB).16 The previous study revealed a functional role of a specific segment of parasite EBL-1 in RBC invasion. Thus, an analogous approach was used in this study to identify additional parasite ligands binding to human glycophorins. Glycophorins, purified from human RBCs, were used as bait to capture the phage display clones from a previously characterized P falciparum complementary DNA library.25 Along with several known malaria ligands such as EBA-175 that bind to glycophorins, we identified multiple overlapping phage clones of MSP1 that bind to immobilized glycophorins (Figure 1A). DNA sequencing revealed a 141-bp region encoding amino acids 204 to 250 located within the N-terminal 83-kDa domain of MSP1 (Figure 1A). In parallel, we also performed several phage display screens using both native and neuraminidase-treated RBCs. An overlapping phage clone of MSP1 containing amino acids 240 to 279, located within the N-terminal 83-kDa domain, was identified (Figure 1A).26 Because the entire 83-kDa domain of MSP1 was difficult to express as recombinant protein in bacteria, 2 smaller segments, termed MSP183A and MSP183B, were expressed. Both overlapping MSP1 phage clones that bound to glycophorins were located within the MSP183A segment (amino acids 20-313), whereas the MSP183B (amino acids 314-669) segment served as a negative control. In addition, we made a GST fusion protein expressing the original MSP1 phage clone 1 (amino acids 204-250) that binds to glycophorins. However, this GST fusion protein, termed MSP15, showed limited solubility and was susceptible to proteolysis (Figure 1B). To overcome this limitation, we scanned the flanking sequences of MSP15 using predictive algorithms of protein secondary structure, and designed a relatively stable MSP112 construct (amino acids 194-296) located within the MSP183A domain of MSP1 (Figure 1B). In the initial binding assays, all recombinant fusion proteins of MSP1 were used; however, the MSP112 construct was found to be most effective in subsequent assays.
Domain organization of recombinant MSP1. (A) MSP1 is grouped into 17 blocks classified as conserved, semiconserved, or variable. The diagram shows 4 subunits of MSP1 following the action of subtilisin-1.26 Two independent overlapping phage clones of MSP1 are indicated. Clone 1, identified in the screen using purified glycophorins, consists of amino acids 204 to 250, whereas clone 2, identified in the screen against neuraminidase-treated RBCs, encodes amino acids 240 to 279. The MSP112 construct incorporates both clones 1 and 2. The respective locations of MSP183A, MSP183B, and MSP119 are also annotated. (B) Coomassie-stained sodium dodecyl sulfate–polyacrylamide gel electrophoresis of MSP1 fusion proteins includes MSP15, containing amino acids 204 to 250 from phage clone 1. MSP112 (amino acids 194-296) expresses a relatively stable protein encompassing both phage clones. MSP183A and MSP183B cover the entire 83-kDa N-terminal subunit of MSP1.
Domain organization of recombinant MSP1. (A) MSP1 is grouped into 17 blocks classified as conserved, semiconserved, or variable. The diagram shows 4 subunits of MSP1 following the action of subtilisin-1.26 Two independent overlapping phage clones of MSP1 are indicated. Clone 1, identified in the screen using purified glycophorins, consists of amino acids 204 to 250, whereas clone 2, identified in the screen against neuraminidase-treated RBCs, encodes amino acids 240 to 279. The MSP112 construct incorporates both clones 1 and 2. The respective locations of MSP183A, MSP183B, and MSP119 are also annotated. (B) Coomassie-stained sodium dodecyl sulfate–polyacrylamide gel electrophoresis of MSP1 fusion proteins includes MSP15, containing amino acids 204 to 250 from phage clone 1. MSP112 (amino acids 194-296) expresses a relatively stable protein encompassing both phage clones. MSP183A and MSP183B cover the entire 83-kDa N-terminal subunit of MSP1.
Interaction of MSP1 with purified GPA and erythrocytes
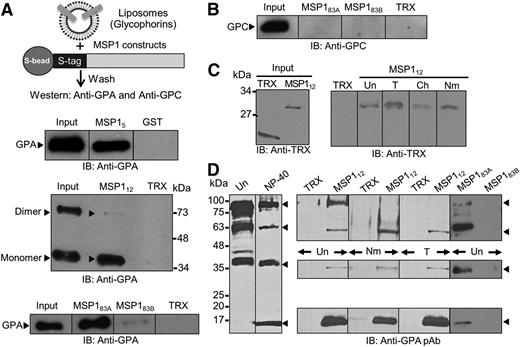
Using recombinant MSP1 segments and purified reconstituted glycophorins, liposome-binding assays were performed to test whether MSP1 interacts with human erythrocyte glycophorins. MSP1 fusion proteins (MSP183A, MSP183B, and MSP112), containing an internal S-tag, were immobilized to S-beads, and the GST-tagged MSP15 segment was bound to glutathione beads. Beads containing the MSP1 fusion proteins were incubated with glycophorins reconstituted as liposomes, and specific binding was quantified along with appropriate negative controls (Figure 2). MSP1 binding to specific glycophorins was detected using antibodies against GPA and GPC. Multiple segments of MSP1, termed MSP15, MSP112, and MSP183A, specifically bound to GPA as compared to control TRX or GST proteins (Figure 2A). In contrast, MSP183B showed minimal but detectable binding with GPA under these conditions, which could originate from the partial overlap of the GPA-binding region of MSP1 extending into the MSP183B segment. Of interest, both MSP15 and MSP183A preferentially pulled down the monomeric form of GPA, whereas the MSP112 segment bound to both monomeric and dimeric forms of GPA (Figure 2B). The purified glycophorins used contain GPA, GPB, and GPC, which served as an additional negative control for the liposome-binding assay. For example, binding of MSP183A was specific to GPA because immunoblotting against GPC did not detect any binding under these conditions (Figure 2B). Moreover, we tested the binding of MSP112 with freshly harvested intact RBCs. The RBC-bound MSP112 was eluted with high salt and detected by immunoblotting against the TRX-tag showing specific interaction between MSP112 and human RBCs (Figure 2C). Pretreatment of RBCs by neuraminidase did not affect their binding affinity for MSP112 (Figure 2C), consistent with the observation that MSP1 clones were originally identified in a phage display screen using neuraminidase-treated RBCs as bait. Surprisingly, MSP112 binding to RBCs was not affected by the pretreatment of RBCs by trypsin, which is known to cleave a large extracellular segment of GPA.17 Together, these data demonstrate that a short segment located within the MSP183A region binds to a neuraminidase- and trypsin-insensitive site (or sites) on human RBCs.
MSP1-GPA binding assays. (A) MSP1 fusion proteins containing an S-tag (GST in MSP15) were immobilized to S-beads (or glutathione beads) and incubated with glycophorin-containing liposomes. Bound proteins were detected by immunoblotting (IB). The TRX fusion protein containing an S-tag and GST were used as negative controls. MSP112 showed specific binding to GPA relative to control in both the monomer (lower band) and dimer species of GPA (upper band). Binding of GPA dimer was detected under mild conditions of beads washings, whereas the GPA monomer binding was observed under both mild and stringent conditions. GPA binding to MSP183A and a very faint signal to MSP183B were also observed. (B) Immunoblotting against GPC did not detect any signal to either MSP183A or MSP183B fusion proteins. (C) RBC binding assay. Bound proteins were eluted by high salt and detected by immunoblotting. MSP112 showed specific binding to RBCs irrespective of their treatment with trypsin (T), chymotrypsin (Ch), or neuraminidase (Nm). Untreated RBCs (Un) were used as control. (D) Detection of GPA species in the detergent-solubilized human RBC ghosts by immunoblotting. Four specific bands of GPA were detected corresponding to homodimer, heterodimer, monomer, and a previously unrecognized 18-kDa band in the NP-40–solubilized ghosts. Immunoblotting using a polyclonal antibody (pAb) against GPA showed specific binding of MSP112 and MSP183A, but not MSP183B, to multiple species of GPA.
MSP1-GPA binding assays. (A) MSP1 fusion proteins containing an S-tag (GST in MSP15) were immobilized to S-beads (or glutathione beads) and incubated with glycophorin-containing liposomes. Bound proteins were detected by immunoblotting (IB). The TRX fusion protein containing an S-tag and GST were used as negative controls. MSP112 showed specific binding to GPA relative to control in both the monomer (lower band) and dimer species of GPA (upper band). Binding of GPA dimer was detected under mild conditions of beads washings, whereas the GPA monomer binding was observed under both mild and stringent conditions. GPA binding to MSP183A and a very faint signal to MSP183B were also observed. (B) Immunoblotting against GPC did not detect any signal to either MSP183A or MSP183B fusion proteins. (C) RBC binding assay. Bound proteins were eluted by high salt and detected by immunoblotting. MSP112 showed specific binding to RBCs irrespective of their treatment with trypsin (T), chymotrypsin (Ch), or neuraminidase (Nm). Untreated RBCs (Un) were used as control. (D) Detection of GPA species in the detergent-solubilized human RBC ghosts by immunoblotting. Four specific bands of GPA were detected corresponding to homodimer, heterodimer, monomer, and a previously unrecognized 18-kDa band in the NP-40–solubilized ghosts. Immunoblotting using a polyclonal antibody (pAb) against GPA showed specific binding of MSP112 and MSP183A, but not MSP183B, to multiple species of GPA.
MSP1 interaction with endogenous GPA
To establish a biochemical interaction between MSP112 and endogenous GPA (GPA), a pull-down assay was performed using human RBC membranes (ghosts). Freshly isolated ghosts contain multiple species of GPA, including the homodimer, heterodimer, and monomer (Figure 2D), consistent with published studies.27 However, after detergent treatment of ghosts, which is required for the pull-down assays, a previously unrecognized 18-kDa species of GPA was observed (Figure 2D, lane with NP-40 treatment).27,28 It is known that solubilization of ghosts in nonionic detergents releases and activates several proteases that may give rise to smaller species of GPA.29 The pull-down assays demonstrated that MSP112 associates with several species of GPA, including the homodimer, heterodimer, monomer, as well as an ∼18-kDa band (Figure 2D). Consistent with this finding, MSP183A, but not MSP183B (Figure 1A), specifically pulled down the same species of GPA (Figure 2D). Whereas the heterodimer, monomer, and 18-kDa species of GPA were pulled down with immobilized MSP112 in the neuraminidase- and trypsin-treated ghosts, the homodimer species of GPA was detected only in the untreated ghosts (Figure 2D). This finding is consistent with the observation that neuraminidase and trypsin treatment of ghosts eliminates the homodimer species of GPA as detected by immunoblotting. Previous studies demonstrated that neuraminidase treatment of RBCs removes the sialic acid content of GPA, and trypsin treatment removes a significant portion of the extracellular domain of GPA.17,30 These 2 treatments, in addition to chymotrypsin, are frequently used to test for receptor enzyme sensitivity on the surface of erythrocytes. GPC is also known to be at least partially sensitive to trypsin treatment that eliminates EBA-140 binding to erythrocytes.31 Of interest, the neuraminidase treatment of intact RBCs appears to increase the relative amount of GPA heterodimer pulled down by MSP112. This observation suggests that the MSP112 interaction with GPA is resistant to neuraminidase treatment and occurs independently of the presence of sialic acids on host GPA.
MSP1 binds to a unique region of human GPA
Although human erythrocyte GPA is considered trypsin sensitive, this treatment is known to leave a short-membrane proximal extracellular segment of GPA on the RBC surface. Trypsin treatment of intact RBCs releases peptides of 31 and 39 amino acids from GPA.17 These 2 peptides correspond to Arg 31 and Arg 39 (Figure 3A), thus leaving ∼40 amino acids exposed on the RBC surface after trypsin treatment. This short ectodomain peptide is contiguous to the single transmembrane domain of GPA consisting of amino acids 72-94. Although the 10F7 monoclonal antibody directed against the extracellular domain of GPA does not recognize the antigen in ghosts prepared from trypsin-treated RBCs, the polyclonal antibody raised against the cytoplasmic C-terminus of GPA recognizes several smaller species of GPA after trypsin treatment of intact RBCs (Figure 3B). We hypothesized that this trypsin-resistant segment of the extracellular domain of GPA is in fact responsible for mediating the binding of MSP112 with RBCs. Guided by the known trypsin cleavage sites of GPA, and combined with a topological model of GPA, we expressed the putative MSP1 binding region of GPA (amino acids 31-72) (Figure 3A), herein referred to as GPA*.17,32 This short segment was expressed as a GST-GPA* fusion protein and used for the binding assays (Figure 3C). The pull-down experiments demonstrated specific binding of MSP112 to the trypsin-resistant extracellular domain of GPA* (Figure 3C). Moreover, to demonstrate specificity, we expressed the N-terminal segment of GPA encoded by amino acids 1 to 30, designated as GST-GPAo. Unlike GST-GPA*, the GST-GPAo fusion protein did not bind to MSP112 (Figure 3C). This observation is consistent with our conclusion that MSP112 interaction with human RBCs is sialic acid independent because the recombinant proteins expressed in bacteria are devoid of sialic acid residues.
Characterization of the MSP1-GPA interaction. (A) GPA, a single transmembrane protein, shows significant glycosylation in the extracellular domain that can be removed by neuraminidase treatment. The approximate locations of the sites recognized by the monoclonal antibody (mAb) and polyclonal antibody (pAb) are indicated. The trypsin cleavage of GPA occurs at 2 distinct arginine residues (Arg31 and Arg39) on the extracellular domain. The extracellular domain of GPA was expressed as 2 nonoverlapping contiguous segments designated as GPAo and GPA*. (B) Both untreated (Un) and trypsin-treated (T) erythrocyte ghosts were tested by immunoblotting (IB) using monoclonal and polyclonal antibodies against GPA. (C) Binding of MSP112 to the trypsin-resistant segment of GPA (GPA*, amino acids 31-72) was detected by immunoblotting against TRX. The trypsin-sensitive segment of GPA (GPAo, amino acids 1-30) was also expressed and tested under identical conditions. Because both fusion proteins contained the GST-tag, GST was also used as a negative control.
Characterization of the MSP1-GPA interaction. (A) GPA, a single transmembrane protein, shows significant glycosylation in the extracellular domain that can be removed by neuraminidase treatment. The approximate locations of the sites recognized by the monoclonal antibody (mAb) and polyclonal antibody (pAb) are indicated. The trypsin cleavage of GPA occurs at 2 distinct arginine residues (Arg31 and Arg39) on the extracellular domain. The extracellular domain of GPA was expressed as 2 nonoverlapping contiguous segments designated as GPAo and GPA*. (B) Both untreated (Un) and trypsin-treated (T) erythrocyte ghosts were tested by immunoblotting (IB) using monoclonal and polyclonal antibodies against GPA. (C) Binding of MSP112 to the trypsin-resistant segment of GPA (GPA*, amino acids 31-72) was detected by immunoblotting against TRX. The trypsin-sensitive segment of GPA (GPAo, amino acids 1-30) was also expressed and tested under identical conditions. Because both fusion proteins contained the GST-tag, GST was also used as a negative control.
Functional role of MSP1-GPA interaction
We tested the activity of MSP183A and MSP112 to inhibit P falciparum invasion of human RBCs. Both N-terminal segments of MSP1 partially inhibited parasite invasion in human RBCs, achieving up to ∼40% inhibition of P falciparum invasion in human RBCs under defined experimental conditions at high protein concentration. This observation is consistent with previous studies providing strong evidence that MSP183 inhibits parasite invasion. For example, in one study, a peptide synthesized within the MSP112 region of MSP183 significantly inhibited RBC invasion.33 In a separate study, antibodies against the MSP183 domain potently inhibited malaria invasion and growth relative to other domains of MSP1.34 Similarly, another study tested the activity of purified erythrocyte glycophorins and demonstrated a significant inhibitory effect on parasite invasion.35 Enzyme treatment of RBCs prior to glycophorin purification altered invasion inhibitory activity of purified glycophorins. Of interest, glycophorins isolated from trypsinized RBCs maintained a significant inhibitory activity, indicating that the trypsin-resistant regions of RBC glycophorins play a significant role in P falciparum parasite invasion of RBCs.35 Thus, the trypsin-resistant region of GPA, as identified in this study as an interacting segment with the MSP112 region, is likely to perform a functional role during malaria parasite invasion.
GPA-deficient band 3 null erythrocytes are resistant to malaria infection
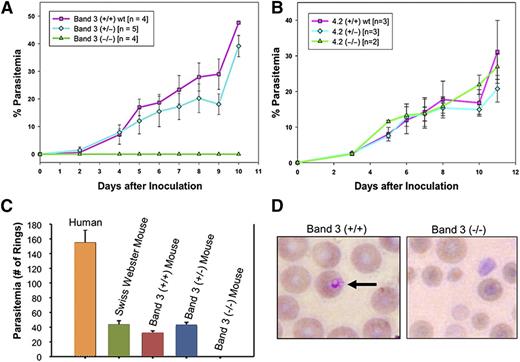
We generated band 3 null mice that were completely deficient in erythroid band 3.21 As a consequence of band 3 loss, the mature RBC membrane also showed a secondary loss of GPA and protein 4.2.21,22 On the basis of our previous findings showing a functional interaction between the carboxyl-terminus of MSP1 and band 3,12 we examined the possibility that RBCs lacking both band 3 and GPA may be resistant to malaria infection. The band 3 null mice lacking the erythrocyte band 3–GPA–protein 4.2 complex are completely resistant to invasion by a virulent strain of murine malaria, P yoelii 17XL (Figure 4A). Band 3 null mice, lacking GPA and protein 4.2 (n = 4), did not display any patent parasitemia, whereas wild-type mice died of infection in 7 to 9 days. The rate of parasite infection in the mutant heterozygous mice was similar to that in wild-type littermates (Figure 4A). To detect any subpatent infection in the band 3 null mice, blood collected from the band 3 null mice on day 14 after inoculation was transferred to wild-type mice (n = 4). None of the subinoculated mice developed malaria infection, indicating that there were no viable parasites present (ie, they were not evident in the peripheral blood smears or flow cytometry) in the band 3 null mice. Further, the serum prepared from the band 3 null mice had no inhibitory effect on the in vitro culture of P yoelli 17XL in wild-type murine RBCs at least for 24 hours, indicating that the cause of the protection from P yoelli 17XL in band 3 null mice is unrelated to the composition of the blood serum. In contrast, protein 4.2 null mice23 did not show any inhibition of parasitemia when challenged with P yoelii 17XL (Figure 4B). These results indicate that deficiency of the band 3–GPA–protein 4.2 complex in the mouse RBC membrane is sufficient for complete resistance to P yoelii 17XL invasion of RBCs. It is noteworthy that a previous study used GPA null mice, which retain erythrocyte band 3, to demonstrate that GPA is not required for lethal P yoelii 17XL infection.36 Therefore, it appears that GPA alone is not essential, but in combination with band 3, this complex is required for malaria infection in vivo. Of interest, the GPA null mice showed reduced parasitemia and improved survival when challenged with Babesia rodhaini, a parasite homologous to malaria, thus indicating a functional role of GPA in Apicomplexan parasite invasion.37
Resistance of band 3–GPA–deficient mice to malaria infection. (A) P yoelii 17XL infection to wild-type (band 3+/+), heterozygous (+/−), and band 3–GPA–deficient (−/−) mice in vivo as described in “Materials and methods.” (B) P yoelii challenge in mice deficient of protein 4.2. (C) Human and mouse RBCs were challenged with P falciparum in vitro and the ring-stage parasites were counted. No invasion of P falciparum was detected in band 3–GPA–deficient (−/−) RBCs during the first cycle of invasion in vitro. (D) Representative images of RBCs from infected wild-type (+/+) and band 3–GPA–deficient (−/−) mice (from panel C) indicate the relative intact morphology of mutant RBCs under the in vitro invasion conditions. Wright-Giemsa was used for staining and images were observed at ×100 magnification. It is noteworthy that the putative regions of P falciparum MSP1 that bind to band 3 (MSP119) and GPA (MSP112) show 48.5% and 43.8% sequence identity, respectively, with their counterparts in P yoelii MSP1.
Resistance of band 3–GPA–deficient mice to malaria infection. (A) P yoelii 17XL infection to wild-type (band 3+/+), heterozygous (+/−), and band 3–GPA–deficient (−/−) mice in vivo as described in “Materials and methods.” (B) P yoelii challenge in mice deficient of protein 4.2. (C) Human and mouse RBCs were challenged with P falciparum in vitro and the ring-stage parasites were counted. No invasion of P falciparum was detected in band 3–GPA–deficient (−/−) RBCs during the first cycle of invasion in vitro. (D) Representative images of RBCs from infected wild-type (+/+) and band 3–GPA–deficient (−/−) mice (from panel C) indicate the relative intact morphology of mutant RBCs under the in vitro invasion conditions. Wright-Giemsa was used for staining and images were observed at ×100 magnification. It is noteworthy that the putative regions of P falciparum MSP1 that bind to band 3 (MSP119) and GPA (MSP112) show 48.5% and 43.8% sequence identity, respectively, with their counterparts in P yoelii MSP1.
To further investigate the role of the host band 3–GPA complex in the P falciparum invasion process, we tested the susceptibility of band 3 null mouse RBCs to P falciparum (3D7 strain) invasion in vitro. When infected with synchronized P falciparum trophozoites, RBCs from both wild-type and band 3 heterozygous mice showed a typical invasion profile after 24 hours postinvasion (Figure 4C), consistent with previous studies.38 However, the band 3 null RBCs lacking both GPA and protein 4.2 did not show any new infection (rings) by P falciparum (Figure 4C). Band 3 null mouse RBCs remained essentially intact in the culture as judged by smear during the course of the experiment (Figure 4D). This observation suggests that the increased fragility of mutant RBCs is unlikely to be the reason for their resistance to malaria infection. To our knowledge, no experimental model system exists where the deficiency of band 3–GPA–protein 4.2 complex can be investigated in structurally normal RBCs.
Discussion
Development of an effective subunit vaccine against malaria requires a precise description of the mechanism by which the merozoites invade host RBCs. The invasion process consists of a sequence of events whereby the merozoite-coat proteins are engaged with host RBC membrane proteins in specific ligand-receptor interactions to form unique invasion pathways. One of the most abundant ligands coating the entire surface of the merozoite is MSP1, a highly conserved protein across multiple parasite species.6 MSP1 is essential for parasite invasion of RBCs, and its genetic disruption is lethal.4,39 The detection of antibodies against the MSP119 segment in an infected host triggered an intense scrutiny of MSP1 as a potential malaria vaccine candidate.8 Despite these efforts, the MSP119-based vaccine efforts have produced only partial protection, suggesting the involvement of other MSP1 domains and/or associated proteins in the invasion process. Therefore, a better understanding of MSP1 interactions is essential for the development of an effective vaccine against malaria.
Early studies on P falciparum MSP1 have shown its interaction with both human and Saimiri monkey RBCs in a sialic acid–dependent manner.40 However, separate studies used peptides derived from P falciparum MSP183, MSP142, and MSP138 domains and demonstrated that these peptides can bind to sialic acid–depleted human RBCs with relatively high affinity.33 Consistent with these findings, our previous work showed that MSP119 interacts with host band 3 receptor, mediating parasite invasion of RBCs via the sialic acid–independent invasion pathway.12,13,41 Host band 3 is the most abundant membrane protein in RBCs and forms a tight stoichiometric complex with GPA. Like band 3, GPA is also highly abundant and serves as a sialic acid–dependent receptor, binding the parasite ligand EBA-175.30,42 On the basis of these observations, we hypothesized that merozoites may display ligands that can potentially bind to band 3 and GPA simultaneously, thus integrating distinct invasion pathways. In this study, we employed several phage display screening strategies to identify a unique sequence located within the N-terminal 83-kDa domain of MSP1 that directly binds to a peptide segment located within the extracellular domain of GPA (Figure 3). MSP112, which includes the GPA-binding sequence, interacts with purified as well as endogenous GPA and recognizes multiple species of GPA (Figure 2). Together, our findings reinforce the possibility that a specific peptide sequence of MSP1 could function as a potent inhibitor of malaria parasite invasion of human RBCs. Of interest, antibodies targeting the trypsin-resistant region of GPA, a region that overlaps with the MSP112 binding site (Figure 3A), caused a pronounced effect on RBC membrane rigidity.43,44 It was concluded that the increase in membrane rigidity requires a ligand-induced interaction with GPA that subsequently alters the RBC cytoskeletal interactions.44 Thus, MSP112 binding to GPA may also alter the RBC membrane properties during parasite invasion. Similarly, a separate study found that reduced RBC membrane deformability on GPA engagement correlated with reduced malaria parasite invasion.45,46 This finding raises the possibility that MSP112 binding to GPA may function by altering the RBC membrane properties during parasite invasion.
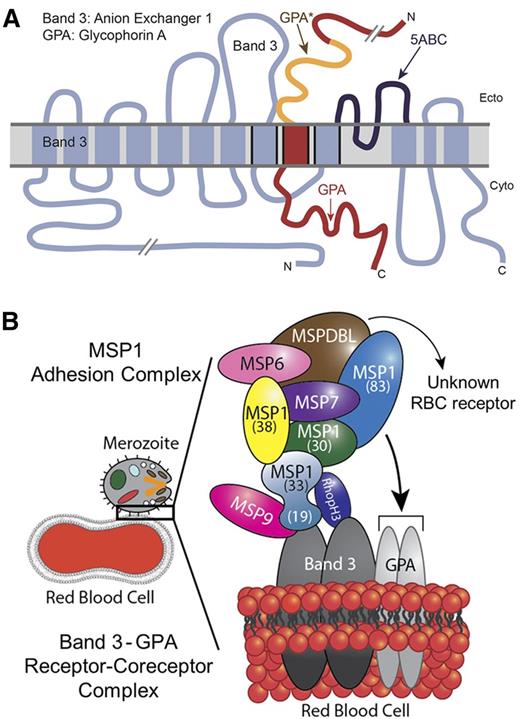
A recent study reported identification of 2 novel proteins associated with MSP1: MSPDBL1 and MSPDBL2.47 However, this study did not detect a direct interaction of MSP1 with human RBCs. In our view, this discrepancy could originate from the protein preparations used in that study. A full-length recombinant MSP1 assembled by tandem tethering of respective MSP1 fragments was used for the RBC binding assays.47 Due to the poor solubility, recombinant full-length MSP1 was subjected to denaturation and renaturation steps, likely resulting in the loss of conformational folding and protein-binding activity. In our experience, recombinant MSP1 is best expressed as soluble subdomains without subjecting them to denaturation-renaturation steps (Figure 1). Given the putative redundancy among parasite-ligand binding partners, it is likely that several interactions occur during the initial merozoite adhesion process, presumably involving multiple host receptors. Indeed, previous studies have predicted a close proximity of band 3 (involving its 12 transmembrane domains) with the transmembrane domain of GPA, and it was shown that Glu-658 of band 3 is required for its association to GPA.32,48 Similarly, topology models predicting the association of band 3 with GPA place the region of band 3, which binds to MSP119, and MSP1-binding region of GPA (GPA*) adjacent to each other within the RBC membrane (Figure 5A).12,48 On the basis of these observations, our results support a model whereby MSP1 and its associated protein complex13 anchors the merozoite on the RBC surface by engaging with 2 distinct closely apposed binding sites located within the surface-exposed domains of band 3 and GPA (Figure 5). This receptor and coreceptor complex is likely to give rise to redundancy while also mediating the adhesion and reorientation steps of the merozoite during invasion.
Model of malaria MSP1–RBC receptor complex. (A) Band 3 and GPA are known to exhibit close physical association in the RBC membrane. The 5ABC region of band 3 that binds to malaria MSP119 (dark blue) is adjacent to GPA* (orange), which binds to MSP112, as adapted from Williamson and Toye.48 (B) Proposed model of malaria merozoite adhesion to erythrocyte by engaging the MSP1 protein complex with host band 3–GPA receptor/coreceptor complex. The recently identified MSPDBL proteins of the MSP1 complex are also shown.47
Model of malaria MSP1–RBC receptor complex. (A) Band 3 and GPA are known to exhibit close physical association in the RBC membrane. The 5ABC region of band 3 that binds to malaria MSP119 (dark blue) is adjacent to GPA* (orange), which binds to MSP112, as adapted from Williamson and Toye.48 (B) Proposed model of malaria merozoite adhesion to erythrocyte by engaging the MSP1 protein complex with host band 3–GPA receptor/coreceptor complex. The recently identified MSPDBL proteins of the MSP1 complex are also shown.47
In P falciparum invasion of RBCs, GPA was identified as the first RBC receptor binding the merozoite EBA-175 in the sialic acid–dependent manner.42,49 Human En(a−) RBCs lacking GPA, and MkMk RBCs lacking both GPA and GPB, confer partial resistance to P falciparum invasion.50-52 A short hairpin RNA–based knockdown GPA in CD34+ stem cells generated GPA-deficient erythrocytes showing partial reduction of P falciparum invasion by the sialic acid–dependent strains.53 In contrast, several previous studies have shown that the majority of the inhibitory effect of GPA on malaria parasite invasion stems from its polypeptide backbone and not its sialic acid content.35,54-56 Using a recombinant trypsin-resistant peptide of GPA that binds to a peptide sequence within MSP183A (Figure 3A), our findings provide definitive evidence that GPA functions as a host receptor via the sialic acid–independent pathway. Future studies will determine how the co-occupancy of GPA by 2 major malaria ligands, MSP1 and EBA-175, is regulated during the RBC invasion process.
Host erythrocyte GPA serves as an important receptor that is necessary, but not sufficient, for malaria parasite invasion of human RBCs. Stable incorporation of GPA in the human RBC membrane requires the presence of band 3 protein, because both mouse and human RBCs genetically lacking band 3 also show a secondary loss of GPA.22,57,58 The existence of a tightly bound band 3–GPA complex in the RBC membrane suggests that the merozoite may exploit this complex to provide some measure of redundancy by switching between the sialic acid–dependent and –independent pathways during invasion. Our findings showing complete resistance of band 3– and GPA-deficient mouse RBCs to malaria infection both in vivo and in vitro (Figure 4) lend further support to this model. Genetic abnormalities of human RBC band 3 are known to provide resistance to malaria infection.57-59 For example, band 3 mutation in Southeast Asian ovalocytosis (SAO) confers partial resistance to malaria infection. Despite multiple explanations including RBC membrane rigidity, cytoskeletal stiffness, and metabolic imbalance, a precise molecular mechanism of malaria resistance in SAO erythrocytes remains poorly understood. Our findings raise the possibility that some of the altered properties of SAO erythrocytes could originate from aberrant GPA interaction with SAO band 3.
Band 3 and GPA constitute the most abundant membrane proteins on the surface of RBCs, whereas parasite MSP1 represents the most abundant merozoite surface protein. This receptor-ligand expression pattern is consistent with the merozoite’s ability to adhere to RBCs regardless of its orientation. Hence, we propose a model in which multiple subunits of MSP1 bind to the band 3–GPA complex during the initial adhesion stage of the invasion process (Figure 5B). This initial adhesion step is then followed by the engagement of additional ligand-receptor interactions, thus facilitating the parasite reorientation and penetration in erythrocytes. On merozoite entry into RBCs, the bulk of the MSP1 complex is released from the merozoite surface. Our model also provides a rationale for the exquisite selectivity of the merozoite’s preference for RBCs in circulation, because the band 3–GPA complex is not expressed in other blood cells.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
The authors thank summer students Brittany Chao, Joshua Cole, and Sheila Amoako for optimizing the phage display screens under the Tufts Building Diversity in Biomedical Sciences program; Faith Goronga and Donna-Marie Mironchuk for their contributions to the drawing of the model cartoon and assembly of figures; Theresa Coetzer for the phage display library; James McKnight of Boston University for help with the design of MSP112 construct; Luanne Peters of The Jackson Laboratory for sharing protein 4.2 null mice; Huiqing Chen and Changling Ma of the University of Illinois for the initial binding assays; Steven Oh and Patrick LeRoy of St. Elizabeth’s Medical Center for assistance with the rodent malaria infection experiments; and the Malaria Research and Reference Reagent Resource Center for some P falciparum strains used in the study.
This work was supported by National Institutes of Health National Heart, Lung, and Blood Institute grants HL060961 and HL095050 and the Tufts Collaborates Seed Grant Program (A.H.C.).
Authorship
Contribution: M.R.B., X.L., T.H., and S.-C.L. performed experiments; M.R.B and A.H.C. wrote the manuscript; and A.H.C. is the principal investigator.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: A. Chishti, Department of Developmental, Molecular & Chemical Biology, Tufts University School of Medicine, 150 Harrison Ave, Room 714, Boston, MA 02111; e-mail: athar.chishti@tufts.edu.


This feature is available to Subscribers Only
Sign In or Create an Account Close Modal