Key Points
Demonstrates that targeting BET bromodomain is a novel strategy to mitigate acute GVHD.
BET bromodomain inhibition regulates DCs and T cells and causes disruption of interaction between BRD4 and acetyl-310 RelA of nuclear factor kappa B.
Abstract
Acute graft-versus-host disease (GVHD) is the major obstacle of allogeneic bone marrow transplantation (BMT). Bromodomain and extra-terminal (BET) protein inhibitors selectively block acetyl-binding pockets of the bromodomains and modulate histone acetylation. Here, we report that inhibition of BET bromodomain (BRD) proteins with I-BET151 alters cytokine expression in dendritic cells (DCs) and T cells, including surface costimulatory molecules, in vitro and in vivo cytokine secretion, and expansion. Mechanistic studies with I-BET151 and JQ1, another inhibitor, demonstrate that these effects could be from disruption of association between BRD4 and acetyl-310 RelA of nuclear factor kappa B. Short-term administration early during BMT reduced GVHD severity and improved mortality in two different allogeneic BMT models but retained sufficient graft-versus-tumor effect. Thus inhibiting BRD proteins may serve as a novel approach for preventing GVHD.
Introduction
Graft-versus-host disease (GVHD) is a major complication of allogeneic bone marrow transplantation (BMT). The priming of donor T cells by antigen-presenting cells and the subsequent proinflammatory cytokine storm and donor T-cell–mediated allogeneic reaction cause target organ damage.1 Evidence suggests that targeting dendritic cell (DC) and/or T-cell function may have therapeutic potential in preventing GVHD.1-4 Bromodomain-containing protein 4 (BRD4) contains 2 tandem bromodomains and an extra-terminal domain and appears to be particularly important, given that it can exert multiple functions by interacting with histone H3, histone H4, and transcription factors by binding to acetyl-lysine residues to regulate target gene transcription.5-11 Recent development of specific inhibitors targeting the acetyl-binding pockets of bromodomain and extra-terminal (BET) family proteins has generated enormous interest for their therapeutic potential.12-14 Although their impact on DCs has not been studied, these inhibitors disrupt the expression of key inflammatory genes, inactivated macrophages, and T cells and exhibit significant anti-inflammatory properties,13,15-18 thus raising the possibility that BET inhibitors may serve as new drugs for the prevention of GVHD.
Study design
Mice and reagents
Female C57BL/6 (B6, H2b) and BALB/C (H2d) mice were purchased from The Jackson Laboratory. All animals were cared for under the regulations of the University of Michigan Committee on the Use and Care of Animals. Bone marrow DCs were generated as previously described.3,19 I-BET151 (Chemie Tek) and JQ1(Sigma-Aldrich, St. Louis, MO) were reconstituted in dimethylsulfoxide (DMSO) and further diluted in phosphate-buffered saline (PBS).
DCs, fluorescence-activated cell sorter, and CFSE labeling analyses
Fluorescein isothiocyanate-, phycoerythrin- or Ag-presenting cell–conjugated monoclonal antibodies to mouse CD11c, CD40, CD80, CD86, programmed death-ligand 1 (PD-L1), major histocompatibility complex class II (MHC II), CD4, CD8a, T-cell receptor β, CD28, phospho-Zap70, H-2Kb, and H-2Kd were purchased from BD Biosciences. Treatment of naïve B6 mice with I-BET for 5 days did not change splenic DC numbers or phenotype (CD80, CD86, CD40) (data not shown). T cells were harvested and isolated from the spleens and purified (>90%) by being negatively isolated using the Pan T Cell Isolation Kit II (Miltenyi Biotec) with an autoMACS separator. T cells were labeled with carboxyfluorescein diacetate succinimidyl ester (CFSE) at a final concentration of 5 μmol/L according to the manufacturer’s instructions (Molecular Probes). Apoptosis of bone marrow (BM) DCs was assessed by Annexin-V/7-aminoactinomycin D staining by fluorescence-activated cell sorter analysis after 8 hours of incubation with DMSO or 1 μM I-BET151. T-cell apoptosis was analyzed after stimulation with anti-CD3/CD28 antibodies following treatment with DMSO or 250 nM I-BET151 for 2 days by Annexin-V staining. The procedures were performed as described previously.3
Enzyme-linked immunosorbent assay, immunoprecipitation (IP), and immunoblotting analysis
Cytokine concentrations in supernatants from cultured DCs treated with or without lipopolysaccharide (LPS; 250 ng/mL) and I-BET151 (500 nm) or JQ1 (100 nm) for 6 hours or from T cells after stimulation and were measured with enzyme-linked immunosorbent assay and read at 450 nm with subtraction at 570 nm by a SpectraMax microplate reader. Cytokine concentrations in sera or supernatants were measured according to the manufacturer’s instructions (BD Pharmingen). IP and immunoblotting analyses were performed as before.7
Quantitative PCR
Total RNA was extracted by using an RNeasy Mini Kit (Qiagen) according to the manufacturer’s protocol. Complementary DNA was synthesized by using a high-capacity cDNA Reverse Transcription Kit (Invitrogen). Real-time polymerase chain reaction (PCR) was performed with SYBR Green PCR mix as described previously.3
Mixed leukocyte reaction
Splenic T cells from BALB/C mice were cocultured with irradiated (25 Gy) B6 DCs at a ratio of 40:1 for 96 hours and pulsed with tritiated thymidine (3H-TdR) for the last 16 hours. The proliferation was determined on a TopCount NTX counter (PerkinElmer).
BMT
BMT was performed as described previously.3,20 Briefly, host mice were irradiated (8.5-10 Gy total body irradiation, 137Cs source) 1 day prior to BMT. Donor BM cells underwent T-cell depletion. T-cell depleted BM cells and T cells (0.25 mL total volume) were injected through the tail veins on day 0. Lethally irradiated B6 or BALB/c recipients were transplanted with 5 × 106 T-cell depleted BM cells and 2 × 106 CD90+ T cells from either syngeneic or allogeneic B6 and BALB/c donors, respectively. Seven doses of I-BET151 (10 mg/kg per day, days −1 to 5) or PBS control were injected intraperitoneally. Survival and GVHD clinical score were monitored over time. For graft-versus-leukemia experiments, briefly, mannose-binding lectin 2 (MBL-2) tumor cells were introduced into the recipients (10 000 per mouse) after conditioning along with donor inoculums on day 0 to model residual leukemia. MBL-2 is a Moloney murine leukemia virus–induced T-cell lymphoma of B6 origin (H2b) and has been extensively used in graft-versus-tumor (GVT) models.21-23 Death was attributed to tumor if tumor was present at necropsy. Death was attributed to GVHD only if no tumor was evident and there was histologic evidence of GVHD. Mice surviving beyond the observation period of BMT were euthanized for histologic evaluation to determine leukemia- and lymphoma-free survival.
Histopathology
Histopathology was performed as described before.3,20-22 Briefly, small and large intestine and liver were harvested on day 7 in each group as indicated (n = 4 mice per group) for detailed histopathologic analysis. Coded slides were scored semiquantitatively by a single pathologist (C.L.) in a blinded manner to assess GVHD-specific pathologic damage.
Results and discussion
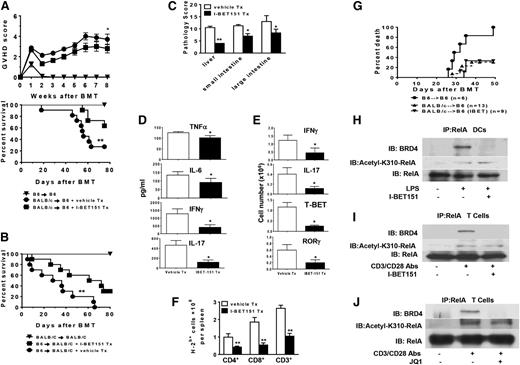
In light of the central role played by nuclear factor kappa B (NF-κB) in inflammatory responses and the anti-inflammatory effects of bromodomain inhibition, we first determined whether activation of DCs and T cells changed the expression of BRD4 and acetylation of the RelA component, which is important for activation of NF-kB.7 Whole-cell lysates were prepared from LPS-treated DCs or CD3/CD28-activated T cells that showed both NF-kB and BRD4 expression in DCs and T cells (Figure 1A-B) and also showed that RelA was acetylated at lysine-310. However, bromodomain inhibition with I-BET151 did not affect RelA acetylation in either activated DCs or T cells (Figure 1A-B).
Impacts of BET bromdomain inhibition on DCs and T cells. (A) RelA was acetylated in LPS-stimulated DCs. Whole-cell lysates were prepared from CD11c microbead–purified DCs treated with or without LPS (250 ng/mL) and I-BET151 (500 nm) for 6 hours as indicated. Western blot was performed to detect expression of BRD4, RelA, and Acetyl-310 RelA. Left panel: Data are representative of 3 experiments with similar results. Right panel: Data represent a summary of 3 independent experiments (mean ± standard error of the mean [SEM]; *P < .001 for comparison of Acetyl-310 RelA between untreated and I-BET151–treated DCs. P values were obtained by two-way analysis of variance [ANOVA]). (B) RelA was acetylated in CD3/CD28-activated T cells. Whole-cell lysates were prepared from T cells after stimulation with CD3/CD28 antibodies and I-BET 151 (250 nm) for 24 hours. Western blot was performed to detect expression of BRD4, RelA, and Acetyl-310 RelA. Left Panel: Data are representative of 3 experiments with similar results. Right panel: Data are a combination of densitometric analyses from 3 independent experiments (mean ± SEM; *P < .001 for the comparison of Acetyl-310 RelA between untreated and I-BET151-treated T cells; P values were obtained by two-way ANOVA). (C-E) Impact of I-BET151 and JQ1 on cytokine expression in DCs. Data shown are the combined results of 3 independent experiments (mean ± SEM; *P < .01). (D) Quantitation of the indicated cytokine messenger RNAs was determined by quantitative real-time PCR. Data are combined from 3 independent experiments (mean ± SEM; *P < .001). (F-G) Impact of I-BET151 in mixed leukocyte reaction. Data were representative of 2 similar experiments (mean ± SEM). (F) Purified BALB/C T cells were labeled with CFSE and cocultured with B6 DCs treated as in (A) and analyzed with flow cytometry. (G) Data are representative of 2 similar experiments. (H) Impact of I-BET151 on surface costimulatory molecules on BM DCs. Data are representative of 3 similar experiments (mean ± SEM) (** P < .01; *** P < .001, one-way ANOVA Dunnett multiple comparison test). (I) Impact of I-BET151 on T-cell proliferation. CFSE-labeled BALB/C T cells were treated as in (B) for 2 days and analyzed by CFSE dye dilution. Data are shown as (left panel) representative or (right panel) combined results from 3 similar experiments (mean ± SEM; **P < .01, Student t test). (J) Impact of I-BET151 on cytokine productions in T cells treated the same as in (B). Cytokines in supernatants were analyzed by enzyme-linked immunosorbent assay. Data were combined from 2 similar experiments (mean ± SEM; *P < .01, Student t test). (K) Impact of I-BET151 and JQ1 on T-cell receptor and CD28 signaling. Purified T cells were stimulated with CD3/CD28 antibodies and treated with I-BET151 (250 nm) or JQ1 (100 nm) for 24 hours and analyzed for expression of CD90.2+TCRβ+, CD90.2+CD28+, and CD90.2+pZAP70+ with flow cytometry. Data are combined from 3 similar experiments (mean ± SEM). (L) Impact of I-BET151 on BM DC viability. Apoptosis analysis after incubation with I-BET151 as described in “Study design.” (M) Impact of I-BET151 on T-cell viability. BALB/C T-cell apoptosis was determined after the cells were stimulated with anti-CD3/CD28 antibodies and treated with I-BET151 for 2 days. Data are from 1 of 2 representative experiments. Abs, antibodies; 7-AAD, 7-aminoactinomycin D.
Impacts of BET bromdomain inhibition on DCs and T cells. (A) RelA was acetylated in LPS-stimulated DCs. Whole-cell lysates were prepared from CD11c microbead–purified DCs treated with or without LPS (250 ng/mL) and I-BET151 (500 nm) for 6 hours as indicated. Western blot was performed to detect expression of BRD4, RelA, and Acetyl-310 RelA. Left panel: Data are representative of 3 experiments with similar results. Right panel: Data represent a summary of 3 independent experiments (mean ± standard error of the mean [SEM]; *P < .001 for comparison of Acetyl-310 RelA between untreated and I-BET151–treated DCs. P values were obtained by two-way analysis of variance [ANOVA]). (B) RelA was acetylated in CD3/CD28-activated T cells. Whole-cell lysates were prepared from T cells after stimulation with CD3/CD28 antibodies and I-BET 151 (250 nm) for 24 hours. Western blot was performed to detect expression of BRD4, RelA, and Acetyl-310 RelA. Left Panel: Data are representative of 3 experiments with similar results. Right panel: Data are a combination of densitometric analyses from 3 independent experiments (mean ± SEM; *P < .001 for the comparison of Acetyl-310 RelA between untreated and I-BET151-treated T cells; P values were obtained by two-way ANOVA). (C-E) Impact of I-BET151 and JQ1 on cytokine expression in DCs. Data shown are the combined results of 3 independent experiments (mean ± SEM; *P < .01). (D) Quantitation of the indicated cytokine messenger RNAs was determined by quantitative real-time PCR. Data are combined from 3 independent experiments (mean ± SEM; *P < .001). (F-G) Impact of I-BET151 in mixed leukocyte reaction. Data were representative of 2 similar experiments (mean ± SEM). (F) Purified BALB/C T cells were labeled with CFSE and cocultured with B6 DCs treated as in (A) and analyzed with flow cytometry. (G) Data are representative of 2 similar experiments. (H) Impact of I-BET151 on surface costimulatory molecules on BM DCs. Data are representative of 3 similar experiments (mean ± SEM) (** P < .01; *** P < .001, one-way ANOVA Dunnett multiple comparison test). (I) Impact of I-BET151 on T-cell proliferation. CFSE-labeled BALB/C T cells were treated as in (B) for 2 days and analyzed by CFSE dye dilution. Data are shown as (left panel) representative or (right panel) combined results from 3 similar experiments (mean ± SEM; **P < .01, Student t test). (J) Impact of I-BET151 on cytokine productions in T cells treated the same as in (B). Cytokines in supernatants were analyzed by enzyme-linked immunosorbent assay. Data were combined from 2 similar experiments (mean ± SEM; *P < .01, Student t test). (K) Impact of I-BET151 and JQ1 on T-cell receptor and CD28 signaling. Purified T cells were stimulated with CD3/CD28 antibodies and treated with I-BET151 (250 nm) or JQ1 (100 nm) for 24 hours and analyzed for expression of CD90.2+TCRβ+, CD90.2+CD28+, and CD90.2+pZAP70+ with flow cytometry. Data are combined from 3 similar experiments (mean ± SEM). (L) Impact of I-BET151 on BM DC viability. Apoptosis analysis after incubation with I-BET151 as described in “Study design.” (M) Impact of I-BET151 on T-cell viability. BALB/C T-cell apoptosis was determined after the cells were stimulated with anti-CD3/CD28 antibodies and treated with I-BET151 for 2 days. Data are from 1 of 2 representative experiments. Abs, antibodies; 7-AAD, 7-aminoactinomycin D.
We next determined the impact of bromodomain inhibition on DC functions. Treatment with I-BET151 significantly reduced the secretion of interleukin-6 (IL-6), tumor necrosis factor α (TNF-α), and IL-12 in the supernatants and expression of messenger RNAs of IL-6 and TNF-α in DCs that were stimulated with LPS (Figure 1C-D). We also observed the reduced expression of IL-6 and TNF-α by JQ1 treatment in DCs after stimulation with LPS (Figure 1E). Pretreatment of B6 DCs with I-BET151 induced significantly reduced expansion of allogeneic BALB/c T cells by both tritium incorporation (Figure 1F) and CFSE tracing (Figure 1G). I-BET151 treatment also markedly reduced the surface expression of CD40, CD80, CD86, PD-L1, and MHC class II in LPS-stimulated DCs in a dose-dependent manner (Figure 1H). These observations collectively show that bromodomain inhibition negatively regulates DCs by reducing surface molecules and T-cell priming and not likely by promoting T-cell exhaustion, given the reduced expression of PD-L1.23
We next examined whether bromodomain inhibition also regulated T cells directly. I-BET151 significantly reduced T-cell proliferation when activated by CD3/CD28 antibodies (Figure 1I). It suppressed the production of IL-17 and interferon gamma (IFN-γ) but increased IL-4 and IL-2 expression, which is consistent with recent reports16 (Figure 1J). Furthermore, following stimulation with CD3/CD28, neither I-BET151 nor JQ1 changed the expression of T-cell receptors (TCRs) and CD28 and the TCR signaling transduction as demonstrated by expression of phospho-Zap70 (Figure 1K). I-BET151 at the doses used in these experiments did not increase apoptosis in either DCs or T cells (Figure 1L-M).
We analyzed the in vivo effects of bromodomain inhibition in MHC-disparate allogeneic BMTs because targeting DCs and/or T cells could mitigate GVHD severity.1-4 In an MHC-disparate BALB/c→B6 BMT model, lethally irradiated B6 mice received 5 × 106 T-cell depleted BM cells and 2 × 106 CD90.2+ T cells from syngeneic (B6) or allogeneic (BALB/C) donors. The allogeneic recipients also received either I-BET151 (10 mg/kg per day) or PBS from days −1 to 5. In dose-finding experiments, all syngeneic animals survived (even at higher doses of 20 and 50 mg/kg), ruling out nonspecific toxicity,whereas the 20-mg/kg dose did not improve GVHD mortality significantly over the 10-mg/kg dose (71% vs 64%; P = not significant). Longer duration of treatment up to day 14 at 10 mg/kg also did not significantly alter survival (data not shown). I-BET151–treated allogeneic animals exhibited significantly reduced GVHD severity and improved survival rates compared with the allogeneic control recipients (Figure 2A). All surviving allogeneic animals demonstrated >95% donor chimerism at end of the observation period, suggesting no clinically significant impact on donor engraftment. Similar results were also observed in another GVHD model, B6→BALB/c, eliminating the possibility of strain-dependent effects (Figure 2B). Significantly reduced histopathologic damage in the small and large bowel and liver (Figure 2C) decreased IL-6, TNF-α, IFN-γ, and IL-17 levels in sera (Figure 2D), decreased absolute numbers of IFN-γ– and IL-17–expressing T cells and T-BET and RAR-related orphan receptor gamma (ROR-γ) –positive T cells (Figure 2E), and decreased total donor T-cell numbers. CD4+ and CD8+ T cells in spleens were observed in the allogeneic animals treated with I-BET151 compared with syngeneic (syn) animals (Figure 2F). However, on day 14, we did not observed all of these alterations (data not shown), suggesting that early I-BET151 administration (days 1 to 5) did not have longer-term effects on T cells after BMT. We next determined whether I-BET151 inhibited GVT effects. I-BET151–treated allogeneic animals demonstrated tumor-induced mortality similar to that of the control allogeneic animals, which shows that a sufficient GVT effect was retained in this model system (Figure 2G).
I-BET151 mitigates GVHD, preserves GVT, and regulates NF-kB. (A) I-BET151 decreases GVHD severity. Lethally irradiated B6 recipients (10 Gy) were transplanted from either syngeneic B6 or allogeneic BALB/C donors and injected with I-BET151 or diluent control as in “Study design.” Survival and GVHD clinical score were monitored over time. Data shown are the combined results of 2 independent experiments (mean ± SEM). Mann-Whitney U test was used for the statistical analysis of clinical scores (*P < .05) and log-rank test was used to compare survival curves (**P < .01). (B) I-BET151 decreases GVHD severity in a second BMT model. BALB/C mice were irradiated (8.5 Gy) and transplanted with 5 × 106 T-cell depleted BM cells and 0.6 × 106 CD90+ T cells from either syngeneic BALB/C or allogeneic B6 donors and treated with I-BET151 as in “Study design.” Survival was monitored over time. Data are combined from 2 independent experiments (n = 10 mice in the allogeneic groups). Log-rank test was used to compare survival curves (** P < .01). (C) Histopathologic analysis of bowel and liver after BMT. The GVHD model and I-BET were treated as in (B). Total GVHD scores are the mean ± SE of the sum of scores (*P < .01). (D) Sera cytokine concentration. Mice were transplanted as in “Study design.” Sera were collected on day +7 from allogeneic animals treated with or without I-BET151 and analyzed for TNF-α, IL-6, IFN-γ, and IL-17. Data shown are combined results in each group (mean ± SE; *P < .01). (E) Impact of I-BET151 on cytokine expression in donor T cells. Spleens were collected on day 7 after BMT. Donor-derived T cells (H2b + CD3+) were stained for intracellular IFN-γ, IL-17, T-BET, and ROR-γ by flow cytometry. Data are from 5 mice per group (mean ± SEM). P values were obtained by Student t test (*P < .01). (F) Impact of I-BET151 on donor T cell (H2b+, CD3+, CD4+, or CD8+) proliferation. Data were obtained by combined numbers from 4 to 5 mice per group (mean ± SEM; *P < .01). (G) Induction of leukemia and lymphoma: Mice were transplanted and injected with MBL-2 tumors and analyzed for tumor-free survival as in “Sudy design.” Data from 2 similar but independent experiments were combined. Survival curve was plotted by using Kaplan-Meier estimates.21-23 (H) Disruption of interaction between Acetyl-K310 RelA and BRD4 in DCs by I-BET151. DCs were treated as in Figure 1A, and whole-cell lysates were processed for IP by RelA antibody. Interaction between acetyl-K310 RelA and BRD4 was analyzed by immunoblotting (IB). Data are representative of 3 similar experiments. (I-J) Disruption of interaction between acetyl-K310 RelA and BRD4 in T cells by (I) I-BET151 and (J) JQ1. Purified T cells were treated with CD3/CD28 antibodies and I-BET151 (250 nm) or JQ1 (100 nm) for 24 hours as indicated. IP was performed as in Figure 2H. Data are representative of 3 similar experiments. Tx, treatment.
I-BET151 mitigates GVHD, preserves GVT, and regulates NF-kB. (A) I-BET151 decreases GVHD severity. Lethally irradiated B6 recipients (10 Gy) were transplanted from either syngeneic B6 or allogeneic BALB/C donors and injected with I-BET151 or diluent control as in “Study design.” Survival and GVHD clinical score were monitored over time. Data shown are the combined results of 2 independent experiments (mean ± SEM). Mann-Whitney U test was used for the statistical analysis of clinical scores (*P < .05) and log-rank test was used to compare survival curves (**P < .01). (B) I-BET151 decreases GVHD severity in a second BMT model. BALB/C mice were irradiated (8.5 Gy) and transplanted with 5 × 106 T-cell depleted BM cells and 0.6 × 106 CD90+ T cells from either syngeneic BALB/C or allogeneic B6 donors and treated with I-BET151 as in “Study design.” Survival was monitored over time. Data are combined from 2 independent experiments (n = 10 mice in the allogeneic groups). Log-rank test was used to compare survival curves (** P < .01). (C) Histopathologic analysis of bowel and liver after BMT. The GVHD model and I-BET were treated as in (B). Total GVHD scores are the mean ± SE of the sum of scores (*P < .01). (D) Sera cytokine concentration. Mice were transplanted as in “Study design.” Sera were collected on day +7 from allogeneic animals treated with or without I-BET151 and analyzed for TNF-α, IL-6, IFN-γ, and IL-17. Data shown are combined results in each group (mean ± SE; *P < .01). (E) Impact of I-BET151 on cytokine expression in donor T cells. Spleens were collected on day 7 after BMT. Donor-derived T cells (H2b + CD3+) were stained for intracellular IFN-γ, IL-17, T-BET, and ROR-γ by flow cytometry. Data are from 5 mice per group (mean ± SEM). P values were obtained by Student t test (*P < .01). (F) Impact of I-BET151 on donor T cell (H2b+, CD3+, CD4+, or CD8+) proliferation. Data were obtained by combined numbers from 4 to 5 mice per group (mean ± SEM; *P < .01). (G) Induction of leukemia and lymphoma: Mice were transplanted and injected with MBL-2 tumors and analyzed for tumor-free survival as in “Sudy design.” Data from 2 similar but independent experiments were combined. Survival curve was plotted by using Kaplan-Meier estimates.21-23 (H) Disruption of interaction between Acetyl-K310 RelA and BRD4 in DCs by I-BET151. DCs were treated as in Figure 1A, and whole-cell lysates were processed for IP by RelA antibody. Interaction between acetyl-K310 RelA and BRD4 was analyzed by immunoblotting (IB). Data are representative of 3 similar experiments. (I-J) Disruption of interaction between acetyl-K310 RelA and BRD4 in T cells by (I) I-BET151 and (J) JQ1. Purified T cells were treated with CD3/CD28 antibodies and I-BET151 (250 nm) or JQ1 (100 nm) for 24 hours as indicated. IP was performed as in Figure 2H. Data are representative of 3 similar experiments. Tx, treatment.
We explored the potential common molecular pathways for the regulatory effects of bromodomain inhibitors in DCs and T cells. NF-κB is crucial for the function of both DCs and T cells, and BRD4 contributes to the maintenance of the NF-κB pathway.7,11,24 However, because bromodomain inhibition did not affect expression of NF-κB or acetylation of RelA (Figure 1A), we hypothesized that I-BET515 treatment modulated DCs and T cells through disruption of the interaction between BRD4 and acetylated RelA. IP studies showed that acetyl-310 RelA was associated with BRD4 in both activated DCs and T cells, whereas treatment with I-BET151 disrupted this association (Figure 2H-I). Similar disruption of this association by JQ1 treatment was also observed in T cells (Figure 2J). This indicates that the disruption of the binding of BRD4 to acetylated RelA by treatment with I-BET151 or JQ1, thus removing the “read” signal of the acetylation-dependent histone code, may be responsible for alterations in DC and T-cell functions, providing the potential mechanistic basis for the preventive role of the BET bromodomain in the inhibition of allogeneic BMT.
Here, we demonstrated that BET bromodomain inhibition by I-BET151 or JQ1 alters cytokine expression in DCs and T cells, represses the expression of surface molecules on DCs, inhibits T-cell expansion, and is associated with disruption of the association between BRD4 and acetyl-310 RelA. Short-term administration of I-BET151 at early periods during BMT can reduce GVHD severity, improve mortality, and preserve the GVT effect. Our studies build on observations by Bandukwala et al16 which demonstrated that another inhibitor, I-BET762, similarly reduced IFN-γ, IL-17, and altered CD4 differentiation. The potential off-target effects of these inhibitors cannot be ruled out, but when previous observations16 with I-BET762 are taken collectively with our observations with I-BET151 and JQ1 effects on T cells or the effects of I-BET151 and JQ1 on DCs, they would suggest that these inhibitors work by targeting bromodomains and/or the off-target effects may be similar. However, because the bromodomains of BET proteins share 75% identity between family members, more selective perturbations of BET protein function may improve therapeutic efficacy in the future.5,6 Our studies suggest that the reduction in GVHD might be a consequence of the multitude of combined immune-regulatory effects on T cells and DCs. However, they do not illustrate which of the effects—reduction of IL-17, IFN-γ, or inflammatory cytokines by DCs—is critical for GVHD. Furthermore, it is possible that the effects on expression of PD-L1 may be precluding complete GVHD prevention.23 It is also possible that these drugs may have other off-target molecular and cellular effects in vivo. Future studies will need to systematically dissect these pathways. Our studies suggest that treatment of short duration was sufficient, possibly because GVHD is induced very early after T-cell transfer in these models, and blunting that initial response mitigates overall severity. Inhibition of only the early events might also explain retention of sufficient immune reactivity to preserve GVT in these models.
Our studies enhance and provide newer insights into histone-dependent epigenetic regulation of immunity3,19,20,25 and suggest that bromodomain inhibition may serve as a novel therapeutic strategy for preventing GVHD while preserving the GVT effect.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
This work was supported by National Institutes of Health grants CA-0173878 and CA143379 (National Cancer Institute), and HL090775 (National Heart, Lung, and Blood Institute) (P.R.).
Authorship
Contribution: Y.S. and Y.W. performed and designed experiments, analyzed data, and wrote the paper; T.T., C.L., N.M., K.O.-W., C.R., E.C., and J.W. performed experiments; D.W. and S.W. analyzed data; and P.R. designed experiments, analyzed data, and wrote the paper.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Pavan Reddy, 3312 CCGC, 1500 E Medical Center Dr, University of Michigan Cancer Center, Ann Arbor, MI 48109; e-mail: reddypr@umich.edu.
References
Author notes
Y.S. and Y.W. contributed equally to this study.

![Figure 1. Impacts of BET bromdomain inhibition on DCs and T cells. (A) RelA was acetylated in LPS-stimulated DCs. Whole-cell lysates were prepared from CD11c microbead–purified DCs treated with or without LPS (250 ng/mL) and I-BET151 (500 nm) for 6 hours as indicated. Western blot was performed to detect expression of BRD4, RelA, and Acetyl-310 RelA. Left panel: Data are representative of 3 experiments with similar results. Right panel: Data represent a summary of 3 independent experiments (mean ± standard error of the mean [SEM]; *P < .001 for comparison of Acetyl-310 RelA between untreated and I-BET151–treated DCs. P values were obtained by two-way analysis of variance [ANOVA]). (B) RelA was acetylated in CD3/CD28-activated T cells. Whole-cell lysates were prepared from T cells after stimulation with CD3/CD28 antibodies and I-BET 151 (250 nm) for 24 hours. Western blot was performed to detect expression of BRD4, RelA, and Acetyl-310 RelA. Left Panel: Data are representative of 3 experiments with similar results. Right panel: Data are a combination of densitometric analyses from 3 independent experiments (mean ± SEM; *P < .001 for the comparison of Acetyl-310 RelA between untreated and I-BET151-treated T cells; P values were obtained by two-way ANOVA). (C-E) Impact of I-BET151 and JQ1 on cytokine expression in DCs. Data shown are the combined results of 3 independent experiments (mean ± SEM; *P < .01). (D) Quantitation of the indicated cytokine messenger RNAs was determined by quantitative real-time PCR. Data are combined from 3 independent experiments (mean ± SEM; *P < .001). (F-G) Impact of I-BET151 in mixed leukocyte reaction. Data were representative of 2 similar experiments (mean ± SEM). (F) Purified BALB/C T cells were labeled with CFSE and cocultured with B6 DCs treated as in (A) and analyzed with flow cytometry. (G) Data are representative of 2 similar experiments. (H) Impact of I-BET151 on surface costimulatory molecules on BM DCs. Data are representative of 3 similar experiments (mean ± SEM) (** P < .01; *** P < .001, one-way ANOVA Dunnett multiple comparison test). (I) Impact of I-BET151 on T-cell proliferation. CFSE-labeled BALB/C T cells were treated as in (B) for 2 days and analyzed by CFSE dye dilution. Data are shown as (left panel) representative or (right panel) combined results from 3 similar experiments (mean ± SEM; **P < .01, Student t test). (J) Impact of I-BET151 on cytokine productions in T cells treated the same as in (B). Cytokines in supernatants were analyzed by enzyme-linked immunosorbent assay. Data were combined from 2 similar experiments (mean ± SEM; *P < .01, Student t test). (K) Impact of I-BET151 and JQ1 on T-cell receptor and CD28 signaling. Purified T cells were stimulated with CD3/CD28 antibodies and treated with I-BET151 (250 nm) or JQ1 (100 nm) for 24 hours and analyzed for expression of CD90.2+TCRβ+, CD90.2+CD28+, and CD90.2+pZAP70+ with flow cytometry. Data are combined from 3 similar experiments (mean ± SEM). (L) Impact of I-BET151 on BM DC viability. Apoptosis analysis after incubation with I-BET151 as described in “Study design.” (M) Impact of I-BET151 on T-cell viability. BALB/C T-cell apoptosis was determined after the cells were stimulated with anti-CD3/CD28 antibodies and treated with I-BET151 for 2 days. Data are from 1 of 2 representative experiments. Abs, antibodies; 7-AAD, 7-aminoactinomycin D.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/125/17/10.1182_blood-2014-08-598037/4/m_2724f1.jpeg?Expires=1767842714&Signature=nehXI~XXAkuE0pwIoOJegg7~ljtjDAJchcMPDwDAlyKPOYiwNF9E0~BCdU5WvxqWgbnGoLhLExDJIa0OwKkMyCkNxUMT9LqisAlWYS96NaBmdyqe7IeVrDa5hlgG2D-PDuV6a9uNPDxouJct3bsn0EB49jkHbxVtPK1nOYBH-TBCXDUxvxxtnEraFXzUpVodMH6ixB8qWvm~vq3~8dafRCyRfuOCTK-89TgpIQA-gMClwGrUj4VB8oWw7yexxQ7l~~wx5xQpqDquNKgYNEUpB~qYLGCwYL1oH8VkleJFI1qvSkaIuZbD7WHEa0M1ad7lcKawWEfPVsUDQdnMe4OXeA__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
This feature is available to Subscribers Only
Sign In or Create an Account Close Modal