Abstract
Macrophages play a critical role in iron homeostasis via their intimate association with developing and dying red cells. Central nurse macrophages promote erythropoiesis in the erythroblastic island niche. These macrophages make physical contact with erythroblasts, enabling signaling and the transfer of growth factors and possibly nutrients to the cells in their care. Human mature red cells have a lifespan of 120 days before they become senescent and again come into contact with macrophages. Phagocytosis of red blood cells is the main source of iron flux in the body, because heme must be recycled from approximately 270 billion hemoglobin molecules in each red cell, and roughly 2 million senescent red cells are recycled each second. Here we will review pathways for iron trafficking found at the macrophage-erythroid axis, with a focus on possible roles for the transport of heme in toto.
Introduction
It is important to begin with the distinctive properties of iron and its role as an essential micronutrient. Iron itself or as a component of heme and iron-sulfur clusters is required for numerous biological processes.1 Additionally, iron’s reactivity makes it quite toxic because it is able to catalyze Fenton reactions and generate dangerous hydroxyl radicals.2 Similarly, imbalances in the biogenesis of iron-containing prosthetic groups, such as heme or iron-sulfur clusters, result in the buildup of toxic intermediates and subsequent pathologies, including anemia or porphyrias. Therefore, the trafficking and transport of iron and iron-containing prosthetic groups must be carefully regulated at cellular and systemic levels.
Iron is unique in how it is absorbed, stored, and mobilized in the body. Dietary iron is not readily absorbed because it is rendered insoluble at intestinal pH and by precipitating compounds found in food, and there are no specific iron excretion mechanisms in the body. Loss of iron can happen via blood loss and via desquamation of intestinal epithelial cells, where it is retained in ferritin when body iron stores are elevated. Women are more vulnerable to iron deficiencies because of monthly loss through menstrual bleeding, as well as through pregnancy and childbirth.
The greatest need for iron mobilization in the body is erythropoiesis. As much as 70% of iron in the adult human body is found in the form of heme in hemoglobin in red blood cells (RBCs).3 Although ∼25 mg of iron is required per day for hemoglobin production and erythropoiesis, only 1 to 2 mg per day is absorbed in the gut. The majority of iron used for red cell formation therefore comes from iron recycled from senescent red blood cells. This review focuses on iron trafficking at both the birth and death of RBCs and the role that macrophages play in these physiological processes. We specifically focus on putative pathways for the transport of heme.
Birth: the role of macrophages during erythropoiesis
Definitive erythropoiesis in humans occurs in the late fetal liver and in adult bone marrow. Hematopoietic progenitors differentiate into early burst-forming units, erythroid, which then further develop into colony-forming units, erythroid. These progenitors continue differentiation through the following stages, each with its own characteristic morphology: proerythroblast, basophilic erythroblast, polychromatic erythroblast, and orthochromatic erythroblast. Orthochromatic erythroblasts enucleate to develop into reticulocytes, which eventually leave the site of erythropoiesis and mature into RBCs in the circulation.4 The process of differentiation from the proerythroblast to the reticulocyte stage has long been known to occur in a niche called the erythroblastic island.5-7 In this niche, developing erythrocytes surround a central macrophage, termed a nurse macrophage, whose contribution to erythropoiesis is still not entirely clear.
Some studies have suggested that the erythroblastic island is essential for definitive erythropoiesis. Liu et al8 analyzed mice that lack palladin, a ubiquitously expressed protein associated with cytoskeletal actin in stress fibers. Unexpectedly, these mice showed compromised ability to form erythroblast islands, a block in definitive erythropoiesis, and death due to anemia around embryonic day 15.5 (E15.5). Importantly, palladin-null erythroid precursors can form erythroblast islands with wild-type macrophages, and hematopoietic stem cells from palladin knockout mice can reconstitute lethally irradiated mice,8 suggesting that palladin might be required only during fetal erythropoiesis. Proteins required for cell-cell interactions between macrophages and erythroid precursors have also been shown to be necessary for definitive erythropoiesis. These include erythroblast macrophage protein in both cell types, vascular cell adhesion molecule 1 in macrophages, and the transcription factor c-Maf, which is required for vascular cell adhesion molecule 1 expression in macrophages.9-11 It has been suggested that β1-, α4-, and α5-integrins might be required on the erythroid cells to interact with macrophages.12-14 Mutants in these proteins exhibit lethal embryonic anemia because of a lack of embryonic definitive erythropoiesis. It is interesting to note that primitive erythropoiesis does not appear to be dependent on the formation of erythroblastic islands.
Paradoxically, other studies have shown that erythroblastic islands are not necessary for effective erythropoiesis in adult mice. In experimental models in which macrophages were depleted by using genetic or chemical means, no severe phenotypes were observed.15-17 When CD169-expressing macrophages were ablated by using diphtheria toxin, bone marrow erythroblast numbers were reduced, but there was no concomitant anemia.15 Similarly, when macrophages were chemically depleted by using clodronate-encapsulated liposomes, mice had reduced hemoglobin concentration and RBC mean cell hemoglobin but did not show a reduction in RBCs.16 However, recovery from erythropoietic stresses (including hemolytic anemia, acute blood loss, and myeloablation) was impaired in these mice. Similarly, blood cell numbers associated with polycythemia could be normalized by the same treatment.
The previously mentioned studies used treatment with toxins to eliminate CD169-expressing macrophages or clodronate liposomes to reduce macrophage numbers via phagocytosis, indiscriminately targeting macrophages in both the bone marrow and spleen. Jacobsen et al18 recently investigated the effect of targeted depletion of bone marrow macrophages on medullary and extramedullary erythropoiesis. Treatment with pharmacologic doses of granulocyte colony-stimulating factor (G-CSF) specifically eliminates resident macrophages in the bone marrow. Jacobsen et al showed that loss of these bone marrow macrophages disrupts the erythroblast niche with a concomitant block in erythropoiesis because mice treated with G-CSF for 4 days showed increased numbers of proerythroblasts and decreased amounts of more differentiated erythroblasts and reticulocytes. Interestingly, G-CSF treatment increased extramedullary erythropoiesis and erythroblastic island formation in the spleen. The effect of G-CSF on erythropoiesis in humans has not been extensively studied, but this treatment has been shown to reduce red cell numbers in humans.19
The authors’ conclusion that the erythroblast island is necessary for erythropoiesis in the bone marrow contradicts the results discussed in the previous paragraph but is supported by the genetic studies mentioned earlier. This inconsistency may be resolved in a number of ways. One could argue that erythroblastic islands are critical during steady-state embryonic definitive erythropoiesis but are only required in adults during stress erythropoiesis. An alternative explanation is that the macrophage depletion treatments used in the earlier experiments were not extensive enough to sufficiently impact red cell production. Further research is needed to establish the requirement for the erythroblastic niche during erythropoiesis and the relative contribution of the bone marrow versus the spleen to blood formation.
Nurse macrophages have been shown to regulate erythroblast development via proliferative and survival signals and to play a role in enhancing enucleation.6,7 Some have suggested that the central macrophage also helps developing erythroid cells by delivering nutrients.7 With grams of iron found as heme in hemoglobin in adult human RBCs, what is the source of this iron, and what role, if any, do nurse macrophages play in delivering iron to erythroid precursors?
Early ferrokinetic studies established that diferric transferrin in the plasma is the sole source of iron for erythroid heme synthesis; the transferrin pathway is shown in Figure 1. Those studies demonstrated that radioactive iron was taken up directly from the plasma, that transferrin was recycled after incorporation of iron into developing reticulocytes, and that a membrane-bound transferrin receptor was required for the process.20-25 The transferrin-iron-transferrin receptor cycle has been extensively studied, showing that diferric transferrin binds the transferrin receptor (also known as CD71) and is internalized via receptor-mediated endocytosis. Iron is released from transferrin following acidification of the endosome, where it can be mobilized for use in the cell, for storage in ferritin, or for export from the cell.26,27
Pathways for delivery of iron and heme iron to developing erythroblasts. Transferrin (Tf) is the major source of iron (red circles) as it emerges from the plasma to bind transferrin receptors (TfRs), becomes internalized, and releases iron into acidic vesicles. Central nurse macrophages found in erythroblastic islands may deliver iron to developing erythroid cells, and secreted ferritin (Ftn) has been observed in the space between nurse macrophages and erythroblasts. Because both developing erythroid cells and macrophages express plasma membrane heme transporters such as Hrg1 and Flvcr1, it is possible that heme (red crosses) itself is transferred to developing red blood cells.
Pathways for delivery of iron and heme iron to developing erythroblasts. Transferrin (Tf) is the major source of iron (red circles) as it emerges from the plasma to bind transferrin receptors (TfRs), becomes internalized, and releases iron into acidic vesicles. Central nurse macrophages found in erythroblastic islands may deliver iron to developing erythroid cells, and secreted ferritin (Ftn) has been observed in the space between nurse macrophages and erythroblasts. Because both developing erythroid cells and macrophages express plasma membrane heme transporters such as Hrg1 and Flvcr1, it is possible that heme (red crosses) itself is transferred to developing red blood cells.
Human patients and mouse models of atransferrinemia have also provided support for the importance of transferrin-bound iron (reviewed in Bartnikas28 ). Humans with mutations leading to the absence of transferrin suffer from hypochromic microcytic anemias. Without the delivery of transferrin-bound iron, erythroid precursors lack sufficient iron to support heme and hemoglobin synthesis. Counterintuitively, there is iron overload in tissues such as the heart and liver because reduced hepcidin increases iron absorption in the gut, and nonerythroid tissues possess transferrin-independent mechanisms for iron uptake.29,30 Several of the mutations responsible for human cases of atransferrinemia have been characterized,31-35 although there are no detailed analyses of the effects of these mutations on transferrin function. The mouse model of atransferrinemia, the hpx mouse, was first described more than 25 years ago.36 The hpx mutation leads to abnormal splicing of the transferrin messenger RNA, resulting in less than 1% of normal levels of the mutant transferrin being detectable.37,38 These hypotransferrinemic mice are born severely anemic and die before weaning if not treated with exogenous transferrin or blood transfusions.
Evidence from atransferrinemic patients and mouse models suggests that transferrin is the sole source of iron used in heme synthesis during erythropoiesis. However, there are many questions remaining with respect to this hypothesis. First, whereas most atransferrinemia patients exhibit symptoms in infancy or early childhood, at least one patient showed no symptoms until adulthood.31 Further characterization of the effect of atransferrinemia-associated mutations on transferrin function would clarify whether the severity and timing of the disease onset correlates with the severity of the transferrin mutation. This might reveal a late-onset human patient with severe transferrin deficiency, indicating alternate pathways for iron transport to developing erythroblasts. Second, the hpx mouse survives until birth. Is this indicative that both primitive and fetal definitive erythropoiesis rely on iron sources other than transferrin-bound iron? Interestingly, mice lacking the transferrin receptor die at E12.5.29 What is the reason for the discrepancy between the two mouse models, which would be expected to have overlapping phenotypes?
There is intriguing evidence for alternate pathways for iron delivery to erythroblasts during their development. Ferritin molecules have been localized between the membranes of the central nurse macrophage and the surrounding erythroblasts (Figure 1, ferritin pathway). Although the exact origin of this ferritin remains a mystery, studies have shown that it is absorbed by erythroblasts by micropinocytosis.39 Cell culture studies have shown that in the absence of transferrin in the media, ferritin synthesized and secreted by macrophages is capable of supporting the differentiation of erythroid precursors.40,41 These results contradict the in vivo evidence that transferrin is the sole means of iron delivery during erythropoiesis. Are these results artifactual? Are they a result of differences in iron homeostasis between an in vitro coculture system and a whole animal model? Notably, an in vitro coculture system would be lacking systemic signals that have been shown to regulate iron homeostasis on an organismal level. The best example of this is hepcidin, a peptide secreted by the liver, which regulates iron release into the circulation by binding to and causing degradation of ferroportin.42-45 Another example is erythroferrone (ERFE), a tumor necrosis factor α superfamily member. ERFE is secreted by erythroblasts in the bone marrow and spleen in response to Jak2/Stat5 signaling induced by erythropoietin, which is mainly secreted by the kidney.46 ERFE signaling suppresses hepcidin expression in the liver enabling the release of iron stores, and this pathway was shown to be important during stress erythropoiesis.46 The effect of ERFE on other iron storage and transport molecules has not yet been elucidated.
There is the possibility that heme itself is transported to developing erythroblasts. The heme importer HRG1 is expressed in early erythroid cells.47 Studies have shown that heme can be exported by macrophages, and macrophages indeed express the plasma membrane heme exporter FLVCR148,49 (Figure 1, heme transport pathway). FLVCR1a, the first reported isoform, was initially discovered as a plasma membrane heme exporter, although recently a second isoform, FLVCR1b, has been shown to function as a mitochondrial heme exporter.48,50,51 Further research is required to determine the precise function of the plasma membrane–localized FLVCR1 protein in erythropoiesis, because an FLVCR1a-specific knockout mouse has not yet been reported. Are there as-yet-undiscovered pathways for the delivery of heme into erythroblasts? Although these pathways are clearly not predominant under physiological conditions, they may play a role in pathological forms of erythropoiesis.
Death: macrophage iron homeostasis during erythrophagocytosis
Human RBCs have a limited lifespan, after which they become senescent and are engulfed and digested by macrophages of the reticuloendothelial system (RES) in a process called erythrophagocytosis (EP) (reviewed in de Back et al,7 Bratosin et al,52 Knutson and Wessling-Resnick,53 Beaumont and Delaby,54 and Ganz55 ). As previously mentioned, the majority of iron required to sustain erythropoiesis is derived from recycled RBCs, and defects in EP lead to aberrant iron metabolism, including anemia and iron overload.
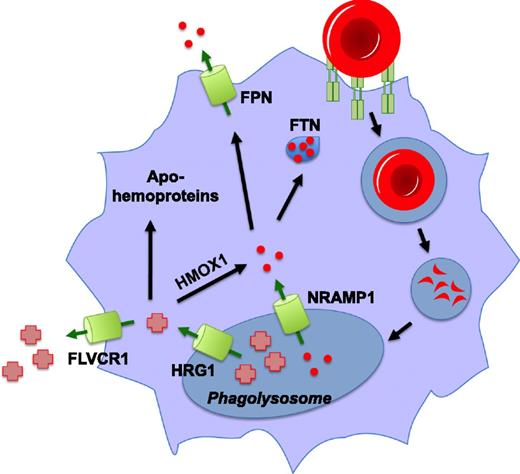
EP occurs when senescent RBCs are recognized by RES macrophages and engulfed via phagocytosis. The relative contribution of splenic versus bone marrow macrophages to erythrophagocytosis is unknown. The RBC-containing phagosome merges with lysosomal vesicles, forming the erythrophagolysosome. Here red cells are degraded, and their contents are subsequently imported into the macrophage cytosol for storage or recycling (Figure 2). The heme catabolism enzyme heme oxygenase 1 (Hmox1) plays a critical role in EP, releasing iron from heme’s protoporphyrin ring for storage or reuse. Mice that lack Hmox1 lose the ability to recycle heme iron and consequently suffer from anemia, reduced serum iron, and accumulation of iron in the liver and the spleen.56 Hmox1−/− mice lack liver and splenic macrophages, which die of heme-associated toxicity, as is observed in vitro when Hmox1−/− macrophages are fed RBCs.57 Heme oxygenase deficiency has been reported in two human patients who also exhibited asplenia and hemolytic anemia.58-60
Pathways for heme and iron recycling during erythrophagocytosis. Senescent red blood cells are recognized by macrophages of the reticuloendothelial system and subsequently internalized and degraded. Iron (red circles) is released from hemoglobin in the phagolysosome by an unknown mechanism, imported into the cytosol via Nramp1, and is either stored in ferritin (FTN) or is exported from the cell by ferroportin (FPN). Heme in the phagolysosome is transported into the cytosol by HRG1, where it can be degraded by HMOX1 for storage or exported as iron. This heme may also be effluxed from the cell by the heme exporter, FLVCR1, and there is some evidence that it can be inserted in toto into apohemoproteins within the macrophage.
Pathways for heme and iron recycling during erythrophagocytosis. Senescent red blood cells are recognized by macrophages of the reticuloendothelial system and subsequently internalized and degraded. Iron (red circles) is released from hemoglobin in the phagolysosome by an unknown mechanism, imported into the cytosol via Nramp1, and is either stored in ferritin (FTN) or is exported from the cell by ferroportin (FPN). Heme in the phagolysosome is transported into the cytosol by HRG1, where it can be degraded by HMOX1 for storage or exported as iron. This heme may also be effluxed from the cell by the heme exporter, FLVCR1, and there is some evidence that it can be inserted in toto into apohemoproteins within the macrophage.
It has been proposed that the heme extracted from hemoglobin during EP was degraded by Hmox1 within the phagolysosome.61 This iron could then be imported into the cytosol by Nramp1, the iron transporter found on the phagolysosomal membrane, for storage in ferritin or export into the circulation by ferroportin. However, there are several problems with the function of Hmox1 in this model. First, studies have revealed that even though Hmox1 is embedded in the ER membrane via a transmembrane segment, the active site of the enzyme is oriented toward the cytosol.62,63 Second, the optimal pH for Hmox1 (pH 7.4) is closer to cytosolic pH than acidic lysosomal pH.64 Third, maximal Hmox1 activity is achieved only in the presence of biliverdin reductase, which is located in the cytosol.65,66 Finally, immunocytochemical studies have shown that heme oxygenases are not found in or on the phagolysosome during EP.67 It is likely that heme must be exported from the erythrophagolysosome before degradation by Hmox1 for iron recycling.
Hrg1, a conserved heme-transporting permease, has recently been shown to mediate heme import from the phagolysosome into the cytosol during EP.68 Hrg1 is expressed in macrophages of the RES and is upregulated transcriptionally and at the protein level in response to treatment with heme or iron and during EP.67,68 This effect was observed in both ex vivo cultured bone marrow-derived macrophages (BMDMs) and in vivo in the spleens and livers of mice injected with heme, damaged RBCs, or the hemolysis agent phenylhydrazine. Hrg1 colocalizes with Lamp1 in endolysosomal compartments and specifically localizes to the erythrophagolysosome during EP.67-69 Transcriptional induction of markers such as Hmox1, Ftn, and Fpn is indicative of increased cellular heme and iron levels in macrophages during EP. Correspondingly, when Hrg1 is depleted, BMDMs are unable to sufficiently upregulate this transcriptional response. This was interpreted as a lack of heme export because the effect was still observed when BMDMs were treated with the iron chelator desferroxamine.68
The same study also showed that a P36L polymorphism in HRG1 is associated with anemia and defective heme transport in a small percentage of African Americans, as determined by expression in a yeast heterologous system, rescue of anemia in zebrafish morphants, and in BMDMs during EP.68 The latter experiments were performed by measuring the biochemical activity of a Golgi-confined hemoprotein reporter horseradish peroxidase (HRP) expressed in BMDMs; increased biochemical activity of HRP was attributed to greater cellular heme availability. Further controls are necessary to definitively show that increased HRP activity was a result of incorporation of recycled heme (rather than heme synthesized de novo from recycled iron). However, these experiments hint that heme derived from recycled RBCs could be incorporated in toto into apohemoproteins rather than be destined for degradation and recycled as iron.
We have previously summarized the evidence for the existence of intracellular heme trafficking pathways in eukaryotic cells.70 We have also identified one such transporter using genetic screens in C. elegans. The ABC transporter mrp-5 was shown to be an intestinal heme exporter essential for worm survival because this nematode is incapable of synthesizing heme and relies exclusively on heme derived from the diet.71,72 In the same study, we showed that fibroblasts lacking the mammalian homolog of MRP5/ABCC5 showed attenuated activity of the Golgi-targeted heme-based HRP reporter in response to exogenous heme. Regardless of whether MRP5/ABCC5 is involved in the subcellular redistribution of heme during erythrophagocytosis in mammals, a network of chaperones and transporters must exist for heme trafficking.
Other studies also hint that not all heme derived from recycled RBCs is degraded into iron. SPI-C is a transcription factor that participates in a differentiation circuit for red pulp macrophages (RPMs) and bone marrow macrophages (BMMs). Initial reports showed that splenic RPMs in Spic−/− mice fail to develop, whereas other populations of monocytes and macrophages remain unchanged.73 Lacking important populations of iron-recycling macrophages, Spic−/− mice consequently develop an iron overload phenotype in the spleen. It was determined that SPI-C expression was activated by heme itself, via derepression by the heme-dependent transcription factor Bach1.74 This heme/Bach1/SPI-C axis regulates the differentiation of monocytes into RES macrophages, for example, in the case of hemolysis and a sudden toxic bolus of heme to iron-recycling macrophages. The same metabolite, although it is toxic to mature RES macrophages, can also stimulate differentiation of monocytes to replace the very population lost as a result of heme toxicity.
We can also ask the following question: What is the source of the heme signal that instructs monocytes to differentiate into RES macrophages? Although Haldar et al74 used either phenylhydrazine treatment or intraperitoneal heme injection to induce SPI-C expression and monocyte differentiation, is it possible that heme derived from recycled RBCs exits the macrophage to induce SPI-C in vivo? Would inhibition of heme efflux from RES macrophages, perhaps via deletion of FLVCR1a from macrophages, mimic a Spic−/− phenotype and prevent differentiation of RPMs and BMMs? Further experimentation will be required to dissect the role of heme as a possible signal molecule after red cell recycling.
Acknowledgments
The authors thank Stefano Rivella for critical discussions and reading of the manuscript.
This work was supported by National Institute of Diabetes and Digestive and Kidney Diseases grants DK85035 and DK74797 (I.H.) from the National Institutes of Health.
Authorship
Contribution: T.K. and I.H. wrote and discussed the manuscript.
Conflict-of-interest disclosure: I.H. is the president and founder of Rakta Therapeutics Inc. (College Park, MD), a company involved in the development of heme transporter-related diagnostics. The remaining author declares no competing financial interests.
Correspondence: Iqbal Hamza, University of Maryland, 2413 Department of Animal and Avian Sciences, Bldg 142, College Park, MD 20742; e-mail: hamza@umd.edu.