In this issue of Blood, Liu et al report on OSU-T315, a new agent that specifically disrupts the phosphatidylinositol 3-kinase (PI3K)/protein kinase B (AKT) pathway and shows high proapoptotic activity against chronic lymphocytic leukemia (CLL) cells, which may indicate a potential therapeutic application in this disease.1
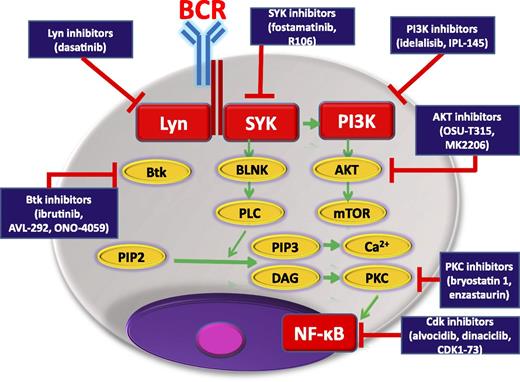
BCR-signaling pathway showing kinase inhibitors, already approved or potentially useful in CLL, and their biological targets. BLNK, B-cell linker; Cdk, cyclin-dependent kinase; DAG, diacylglycerol; Lyn, member of the Src family of protein tyrosine kinases; NF-κB, nuclear factor κB; PIP2, phosphatidylinositol 4,5-bisphosphate; PIP3, phosphatidylinositol (3,4,5)-trisphosphate; PKC, protein kinase C; PLC, phospholipase C; mTOR, mammalian target of rapamycin.
BCR-signaling pathway showing kinase inhibitors, already approved or potentially useful in CLL, and their biological targets. BLNK, B-cell linker; Cdk, cyclin-dependent kinase; DAG, diacylglycerol; Lyn, member of the Src family of protein tyrosine kinases; NF-κB, nuclear factor κB; PIP2, phosphatidylinositol 4,5-bisphosphate; PIP3, phosphatidylinositol (3,4,5)-trisphosphate; PKC, protein kinase C; PLC, phospholipase C; mTOR, mammalian target of rapamycin.
Great progress has been made in the diagnosis and treatment of CLL, but this type of leukemia still remains incurable, and the introduction of new drugs and new therapeutic strategies are still awaited. For the last 20 years, significant progress in molecular biology has resulted in better characterization and understanding of the biology and prognosis of CLL. Accumulating evidence supports the critical role played by B-cell receptor (BCR) activation in the pathogenesis of CLL and has provided new opportunities for the development of innovative, more effective therapies. Recently, several small molecular kinase inhibitors targeting the proximal BCR signaling pathway, including Bruton’s tyrosine kinase (BTK) inhibitor, ibrutinib, and phosphatidylinositol 3-kinase p110δ (PI3Kp110δ) inhibitor, idelalisib, have been developed (see figure). These drugs are part of a promising new strategy for the effective targeted treatment of CLL and have been recently approved by the US Food and Drug Administration.2,3
Among the new therapies intended for CLL, much attention is being paid to the implementation of agents with a high potential for triggering apoptosis of tumor cells.4 The PI3K/AKT signaling pathway plays a pivotal role in regulating multiple cellular events supporting the survival of CLL B cells. In CLL, activation of the PI3K/AKT pathway has an important role in the generation of the proliferative pool, and the pharmacological compounds that disrupt this pathway may have significant antiproliferative activity.5 Studies based on the coculture of CLL cells with marrow stromal cells show that PI3K/AKT signaling plays an important role in the activation of both cell types during their interactions that support survival of CLL tumor cells.6 OSU-T315 is an inhibitor of the PI3K/AKT pathway, which exhibits high in vitro potency against a panel of prostate and breast cancer cell lines.7 Liu et al report their results of a study comparing the activity of OSU-T315 in both CLL-derived cell lines and primary CLL cells with its action in normal lymphocytes. They document a unique mechanism of OSU-T315 action. Namely, the compound directly abrogates AKT signaling by preventing the translocation of AKT kinase into lipid rafts. Importantly, in this mechanism, the activation of receptor-associated kinases remains unaltered. As a consequence, OSU-T315 induces caspase-dependent apoptosis by suppressing BCR, CD49d, CD40, and Toll-like receptor 6-mediated AKT activation in CLL cells. This mechanism is independent of the integrin-link kinase.1 Moreover, in a transplant TCL1 mouse model, OSU-T315 prolongs the survival of leukemic mice by the selective targeting of CLL cells and sparing of normal B or T lymphocytes.
In addition to OSU-T315, other AKT inhibitors exert antileukemic activity. The best sample is MK2206, a highly selective oral allosteric AKT inhibitor found to exert the in vitro efficacy of MK2206 on CLL B-cell survival.8 MK2206 abolishes phosphorylation of AKTS473, significantly decreasing the activation of the AKT target, p70S6K, without affecting the p85 unit of PI3K, and in turn activates caspase-dependent apoptosis of CLL cells. Moreover, MK2206 selectively inhibits BCR-induced CCL3, CCL4, CCL2, and interleukin-2Rα production, most probably by abrogating AKT and extracellular signal-regulated kinase. Importantly, MK2206 kills CLL cells and spares healthy lymphocytes, in a similar way to OSU-T315.8
Ibrutinib and idelalisib have been found to demonstrate a remarkable clinical response in CLL patients. However, recent studies have identified patients refractory to these agents and characterized a BTK mutation (BTKC481S) responsible for ibrutinib resistance.9,10 A way of overcoming the ibrutinib resistance demonstrated by these patients is urgently needed. Recent studies suggest that therapeutic intervention with other kinase inhibitors, such as idelalisib or spleen tyrosine kinase (SYK) inhibitors, may shut down BCR signaling and inhibit CLL proliferation in patients who have acquired the BTKC481S mutation.9 However, these drugs inhibit the BCR signaling pathway at proximal kinases, and some CLL patients can be refractory to both ibrutinib and idelalisib. For those patients, compounds such as OSU-T315, which directly abrogates AKT activation without altering the activation of receptor-associatedkinases, can be particularly useful.
In conclusion, OSU-T315 is a new compound showing a specific, novel mechanism of abrogating the AKT pathway and inhibition of BCR activity in the leukemic cell. Therefore, this compound can be potentially useful in the treatment of high risk CLL, including patients with del(17p13.1), unmutated IGVH, or those who may be resistant to ibrutinib.
Conflict-of-interest disclosure: The authors declare no competing financial interests.