In this issue of Blood, Jing et al have identified 2 novel pathways involved in the dexamethasone response in pediatric B-precursor acute lymphoblastic leukemia (ALL).1
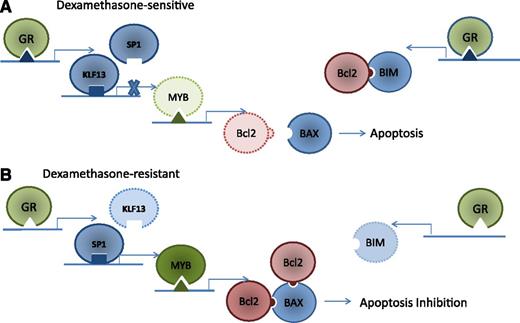
GR-mediated induction of apoptosis in dexamethasone-sensitive ALL. (A) The GR receptor binds to a novel GR promoter of KLF13, activating KLF13 binding to MYB promoter and displacing the MYB activator SP1 (left). KLF13 deactivates MYB expression, decreasing the expression of Bcl2 and allowing BAX to trigger apoptosis. Concurrently, GR also binds a GR promoter in the BIM intronic region (right), increasing BIM expression. BIM binds existing Bcl2, preventing BAX binding and resulting in BAX-mediated apoptosis. (B) Both the GR promoter in KLF13 and the intronic BIM GR promoter are absent in patient-derived leukemia cells resistant to dexamethasone, allowing Bcl2 binding to BAX, resulting in apoptosis inhibition.
GR-mediated induction of apoptosis in dexamethasone-sensitive ALL. (A) The GR receptor binds to a novel GR promoter of KLF13, activating KLF13 binding to MYB promoter and displacing the MYB activator SP1 (left). KLF13 deactivates MYB expression, decreasing the expression of Bcl2 and allowing BAX to trigger apoptosis. Concurrently, GR also binds a GR promoter in the BIM intronic region (right), increasing BIM expression. BIM binds existing Bcl2, preventing BAX binding and resulting in BAX-mediated apoptosis. (B) Both the GR promoter in KLF13 and the intronic BIM GR promoter are absent in patient-derived leukemia cells resistant to dexamethasone, allowing Bcl2 binding to BAX, resulting in apoptosis inhibition.
Using patient-derived xenografts, the authors show that the glucocorticoid receptor (GR) coordinately regulates both the antiapoptotic protein Bcl2 and the proapoptotic protein BIM (see figure). Using gene expression profiling, chromatin immunoprecipitation (ChIP), and ChIP sequencing (ChIP-seq) analysis, the authors identified a novel intronic GR binding site within the BIM coding region. The group also showed that both the GR promoter in KLF13 and the intronic BIM GR promoter were absent in leukemia cells resistant to dexamethasone.
As with most accounts of apoptosis regulation, GR regulation of apoptosis in leukemia cells is both elegant and complex. In dexamethasone-sensitive patient-derived xenografts, Bcl2 is indirectly mediated through KLF13 and MYB (panel A), resulting in decreased production of Bcl2. In addition, GR increases the expression of BIM, resulting in Bcl2 inhibition. In dexamethasone-resistant leukemia cells, the GR does not hinder Bcl2 binding to BAX, resulting in an inhibition of apoptosis (panel B). Thus, the authors identified novel mechanisms for the GR to both decrease Bcl2 production and inhibit Bcl2 function.
The results of this article are compelling for 2 reasons. First, the authors use a clinically relevant model of pediatric ALL xenografts. The leukemia cells used for this analysis are cells derived from pediatric patients with ALL that have been expanded and maintained in immunodeficient NOD/SCID mice. These cells are more likely to have maintained the growth characteristics and signal transduction pathways of leukemia patients than established cell lines are.
Second, the results have clinical implications. Sensitivity to glucocorticoids is one of the main predictors of ALL chemosensitivity and is an indicator of drug responsiveness in many pediatric clinical trials.2,3 Approximately 10% of patients with ALL have a poor response to steroid treatment, a characteristic associated with significantly inferior outcome.3 Glucocorticoid resistance in leukemia cell lines is often due to the absence of the GR; however, many patient-derived leukemia cells with glucocorticoid resistance have functional GRs.4 The mechanisms of glucocorticoid resistance in patient-derived samples has been previously linked to Bcl2, BIM, and Mcl1.5,6 This article provides further insight into how Bcl2 regulates glucocorticoid resistance and suggests that GR resistance could be mediated through absent intronic GR promoters in BIM or the absence of GR promoters for KLF13.
Strategies that reverse glucocorticoid resistance could have a significant impact in increasing overall clinical survival, particularly for high-risk leukemia groups such as infants with the MLL translocation and patients with NOTCH-mutated T-cell ALL. Agents known to increase glucocorticoid sensitivity, such as proteasome inhibitors, are currently undergoing clinical trials for pediatric ALL in the United States (#NCT02112916). This research work is particularly interesting when considered with studies showing that proteasome inhibition can increase BIM expression in chronic lymphocytic leukemia (CLL) by inhibiting BIM degradation, leading to enhanced CLL apoptosis in patient CLL cells that are treated with both dexamethasone and the proteasome inhibitor MG132.7
Several strategies have been explored to enhance the glucocorticoid-mediated apoptosis of leukemia cells in preclinical studies, including use of mTOR inhibitors,8 glycolysis inhibitors,9 and γ-secretase inhibitors.10 Despite a slow start due to liver toxicity in earlier clinical trials, several γ-secretase inhibitors are currently in early-stage clinical trials for solid tumors and T-cell ALL. Restoring glucocorticoid sensitivity could not only improve clinical outcome in high-risk groups with newly diagnosed ALL but also improve response rates in patients with difficult-to-treat relapsed ALL. As novel treatment strategies are developed to improve ALL survival, it may be helpful to revisit and perhaps reinvigorate chemotherapy agents that have a proven track record in ALL. Thus, novel treatment strategies may improve ALL survival by reinvigorating one of the most commonly used and effective chemotherapy agents in ALL.
Conflict-of-interest disclosure: The author declares no competing financial interests.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal