In this issue of Blood, Kurtz et al report the potential clinical utility of immunoglobulin high-throughput sequencing as a tool for disease monitoring and surveillance in aggressive B-cell lymphoma.1
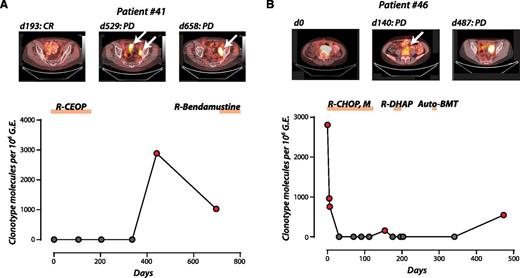
Ig-HTS for disease progression or relapse in DLBCL in 2 patients. (A) A patient treated for limited stage DLBCL achieved a complete response and was shown on monitoring for plasma tumor DNA to have relapse before CT/PET detection of recurrent disease (lead time = 88 days). (B) A patient with 2 separate relapses detected by Ig-HTS coincident with radiologic relapse. Reprinted from Figure 5A-B in the article by Kurtz et al that begins on page 3679.
Ig-HTS for disease progression or relapse in DLBCL in 2 patients. (A) A patient treated for limited stage DLBCL achieved a complete response and was shown on monitoring for plasma tumor DNA to have relapse before CT/PET detection of recurrent disease (lead time = 88 days). (B) A patient with 2 separate relapses detected by Ig-HTS coincident with radiologic relapse. Reprinted from Figure 5A-B in the article by Kurtz et al that begins on page 3679.
Posttreatment surveillance in diffuse large B-cell lymphoma (DLBCL) has typically included clinic visits every ∼3 months, with interval imaging every 6 months, using either computed tomography (CT) or CT/positron emission tomography (PET). This approach is recommended in current National Comprehensive Cancer Network guidelines. Recent studies have challenged this, suggesting that routine surveillance imaging has a low yield for detecting relapse in asymptomatic patients, has no effect on overall survival, and is not cost effective.2-4 Additionally, the high rate of false-positive results, particularly for functional imaging, leads to increased anxiety and unnecessary interventions, including biopsies, added to which are the potential risks of radiation exposure for frequent imaging.5,6 Currently, we do not have a reliable biomarker of disease in this clinical context. Similarly, response assessment during and after therapy has relied primarily on imaging studies that lack predictive value, limiting our ability to explore response-adapted strategies in these diseases.
The report from Kurtz et al describes the potential for high-throughput sequencing of tumor-specific immunoglobulin genes (Ig-HTS) in the blood as a noninvasive test to monitor disease response and recurrence in DLBCL.
There are several aspects of this study that suggest that this technique could have clinical utility:
The investigators were able to detect an informative clonotypic sequence in 83% of patients in this study. Not surprisingly, the yield was higher for those with fresh frozen vs formalin-fixed paraffin-embedded tissue and also for those with more tissue available, but even in their real-world validation cohort, a clone was detected in 71% of patients. As long as sufficient paraffin-embedded tissue was available from an excisional biopsy, the chances of identifying a clonotype were high. Importantly, the rate of detection did not appear to be related to stage at presentation, so this technique has potential even in those patients with limited stage disease.
Detection of molecular disease in the plasma was consistently more sensitive than in circulating tumor cells and was also a more reliable marker of disease, at least in the relapse setting, than serum lactate dehydrogenase (LDH). At present, serum LDH is probably the most widely used serum tumor marker in aggressive B-cell non-Hodgkin lymphoma, but its utility in this context has never been validated prospectively. In this study, the authors demonstrate that plasma levels of tumor DNA correlated more closely with tumor volume than did serum LDH.
In general, sequential levels of circulating tumor DNA closely followed changes in tumor volume, assessed by functional imaging. Escalating levels predicted radiologic and clinical relapse or occurred concurrently with clinical relapse in most patients. The figure demonstrates 2 illustrative patients in which increased levels of circulating tumor DNA correlated with relapse/progression on CT/PET, with varying lead times.
In the posttreatment surveillance setting, the specificity of molecular disease detection in the plasma was 100%. This suggests that measurement of circulating tumor DNA overcomes the major limitation of functional imaging in the surveillance context: the unacceptably high false-positive rate.
Although these preliminary data are promising, there are some limitations in the present study. The sample size is quite small and is heterogeneous with respect to disease subtype and treatment. The analyses of the relationship between functional imaging and tumor DNA were not adjusted for treatment: this will be important to investigate in future studies because existing data suggest that the predictive value of functional imaging may be treatment dependent.7 Although the authors conducted a real-world validation of their ability to detect clonotypic sequences, it’s not clear how the success rate of this technique will vary between laboratories, and how reproducible the results will be across different centers. In view of the small sample size, the authors were not able to investigate the potential influence of disease-related factors such as molecular subtype: this will be an important question to address in future studies.
Whether Ig-HTS will prove to be a real advance in the setting of response assessment and response-adapted therapy is unclear. Early assessment of response will prove beneficial only if there is an effective second-line therapy for nonresponders. It will be important to demonstrate that earlier detection of relapse translates to an improved outcome for patients. As described in a previous report, this technique might also be applicable to the detection of minimal residual disease (MRD).8 The use of MRD to guide therapy has, until now, not been evaluated in DLBCL because of the lack of a reliable assay using peripheral blood.
Where this technique is likely to have the most immediate impact is in the posttreatment surveillance setting. Results from this study suggest that Ig-HTS in plasma is a noninvasive investigation with high specificity, closely correlated with tumor burden, and demonstrates a temporal relationship to radiologic and clinical relapse. The lead time associated with molecular detection may not have a major impact on subsequent survival, but the preliminary data in this study suggest that HTS may replace routine imaging or at least restrict its use to those patients in whom the suspicion for recurrence is highest. This will limit radiation exposure and reduce the unnecessary anxiety and interventions that accompanying false-positive scans. If the results of this preliminary study are confirmed, it will change practice.
Conflict-of-interest disclosure: J.W.S. has received consulting fees and honoraria from Seattle Genetics.