In this issue of Blood, Schwartz et al identify the nuclear factor (NF)-κB/Bcl-xL pathway as critical for regulation of eosinophil survival in inflammatory conditions.1
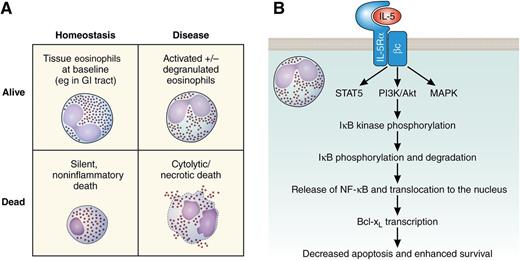
Eosinophils. (A) Schematic representation of consequences of eosinophil life or death in homeostasis and disease. (B) Schematic signal transduction pathway leading to IL-5–mediated eosinophil survival. Bcl-xL, B-cell lymphoma–extra large; βc, common β chain; GI, gastrointestinal; IκB, inhibitor of NF-κB; IL-5Rα, IL-5 receptor α; MAPK, mitogen-activated protein kinase; PI3K, phosphatidylinositol 3-kinase; STAT5, signal transducer and activator of transcription 5.
Eosinophils. (A) Schematic representation of consequences of eosinophil life or death in homeostasis and disease. (B) Schematic signal transduction pathway leading to IL-5–mediated eosinophil survival. Bcl-xL, B-cell lymphoma–extra large; βc, common β chain; GI, gastrointestinal; IκB, inhibitor of NF-κB; IL-5Rα, IL-5 receptor α; MAPK, mitogen-activated protein kinase; PI3K, phosphatidylinositol 3-kinase; STAT5, signal transducer and activator of transcription 5.
Cell death is essential for many physiologic processes, and its dysregulation characterizes numerous human diseases. This is especially true for eosinophils, because these cells do not undergo substantial extramedullary hematopoiesis, yet their tissue levels can be markedly and selectively increased by a combination of recruitment from the blood and regulation of their cell death within tissues. The field of eosinophil survival has come a long way since the original finding that human blood–derived eosinophils had prolonged survival (>14 days) when cocultured with endothelial cells and with the subsequent identification of interleukin (IL)-5, granulocyte macrophage–colony-stimulating factor (GM-CSF), and IL-3 as key eosinophil survival factors.2 It is now appreciated that these eosinophil-directed hematopoietins inhibit eosinophil apoptosis and that IL-5 has a special capacity to promote eosinophil development, which has led to several therapeutics that lower eosinophils by blocking IL-5, such as mepolizumab and reslizumab, which are at advanced stages of development.3
However, whether eosinophils are better “dead or alive” is still an unresolved question. Simplistically, both living and dead eosinophils can lead to positive and negative outcomes (see figure). For instance, eosinophils are homeostatically present in some tissues with no ill effect (figure panel A, upper left)4 and have been shown to contribute to a variety of homeostatic functions, including production of secretory immunoglobulin A in the intestine.5 Yet during many eosinophilic inflammatory diseases, eosinophils display an activated phenotype and likely induce tissue damage (figure panel A, upper right).6 Eosinophil cell death was previously viewed dichotomously, as either noninflammatory apoptotic (figure panel A, lower left) or proinflammatory necrotic (figure panel A, lower right) cell death, but recent studies have identified additional cell death pathways, including regulated necrosis, necroptosis, and autophagy-associated cell death.7,8 Importantly, especially in situations when one cell death pathway is inhibited, other pathways can be activated. Current research in the field focuses on the goal of preventing the destructive forms and encouraging the silent forms of cell death. Understanding the mechanisms of regulating different types of eosinophil cell death is thus critical for moving the field forward.
In this issue, Schwartz et al deepen our understanding of the mechanism by which eosinophils accumulate in tissues. In particular, the authors demonstrate that in addition to regulating eosinophil development, IL-5, along with GM-CSF and IL-3, mediates eosinophil cellular survival by NF-κB–induced Bcl-xL, which inhibits apoptosis. This conclusion was based on a variety of pharmacologic inhibitor studies, as well as on the development of a novel, eosinophil-specific Cre recombinase “deleter” mouse line. This mouse line enabled them to delete the Nfkbia gene (which encodes IκBα) specifically in eosinophils, thereby constitutively activating the NF-κB pathway in eosinophils. Although this eosinophil-specific, constitutive activation did not increase eosinophil numbers at baseline, it did increase eosinophil levels after experimental helminthic infection. Using a combination of in vitro and in vivo approaches, the authors further delineated the pathway to show that NF-κB activation leads to transcriptional expression of antiapoptotic Bcl-xL and, ultimately, to increased survival (figure panel B); despite most of the studies being limited to mice, this latter finding was shown to also apply to human eosinophils, adding translational significance.
Many questions remain to be answered in future studies. For instance, what is the effect on disease outcomes? As outlined above, it is not straightforward to predict the consequence of inhibiting a single cell death pathway. If apoptosis is inhibited, will eosinophils “live long and prosper” or die a more violent death, such as via regulated necrosis? Future studies will also address some of the limitations of the current study. For instance, the exact identification of the cell death pathway (apoptosis, regulated necrosis, and so forth) will need to be established, because the authors focused on annexin V/propidium iodide–positive cells; both of these dyes gain access to and stain cells after the membrane is permeabilized, irrespective of the cell death pathway that leads to it. Additionally, the preponderance of double-positive cells may be an artifact of the in vitro nature of the experiments, wherein the physiologic mechanisms for removal of apoptotic cells are not present and cells may undergo secondary necrosis, or may suggest a different pathway of cell death. Furthermore, the Cre recombinase is expressed in only 20% to 30% of eosinophils in the present study. Another strain of eosinophil-specific Cre–expressing mice, which was developed recently by Doyle et al,9 has 100% Cre expression in eosinophils. Having 2 different strains would give investigators the opportunity to tailor strain selection to the needs of the experimental question.
In summary, Schwartz et al have shown that IL-5 mediates eosinophil production by promoting eosinophil differentiation and also by inhibiting cellular apoptosis. Although this conclusion is not entirely novel, the authors deepen our understanding by showing that the action of IL-5 is dependent on the activation of NF-κB and the subsequent induction of the antiapoptosis protein Bcl-xL. Thus, the eosinophil lives again!
Conflict-of-interest disclosure: M.E.R. is a consultant for Immune Pharmaceuticals, Celsus Therapeutics, Novartis, Receptos, and NKT Therapeutics; has an equity interest in Immune Pharmaceuticals, Celsus, and Receptos; is an inventor of patents owned by Cincinnati Children’s Hospital Medical Center; and has a royalty interest in reslizumab, a drug being developed by Teva Pharmaceuticals. N.Z. declares no competing financial interests.