Key Points
The bispecific, tetravalent antibody AFM13 represents a new approach engaging natural killer cells via CD16A to fight CD30+ malignancies.
AFM13 is well tolerated and active in Hodgkin lymphoma patients who received all standard therapies, including brentuximab vedotin.
Abstract
AFM13 is a bispecific, tetravalent chimeric antibody construct (TandAb) designed for the treatment of CD30-expressing malignancies. AFM13 recruits natural killer (NK) cells via binding to CD16A as immune effector cells. In this phase 1 dose-escalation study, 28 patients with heavily pretreated relapsed or refractory Hodgkin lymphoma received AFM13 at doses of 0.01 to 7 mg/kg body weight. Primary objectives were safety and tolerability. Secondary objectives included pharmacokinetics, antitumor activity, and pharmacodynamics. Adverse events were generally mild to moderate. The maximum tolerated dose was not reached. Pharmacokinetics assessment revealed a half-life of up to 19 hours. Three of 26 evaluable patients achieved partial remission (11.5%) and 13 patients achieved stable disease (50%), with an overall disease control rate of 61.5%. AFM13 was also active in brentuximab vedotin–refractory patients. In 13 patients who received doses of ≥1.5 mg/kg AFM13, the overall response rate was 23% and the disease control rate was 77%. AFM13 treatment resulted in a significant NK-cell activation and a decrease of soluble CD30 in peripheral blood. In conclusion, AFM13 represents a well-tolerated, safe, and active targeted immunotherapy of Hodgkin lymphoma. A phase 2 study is currently planned to optimize the dosing schedule in order to further improve the therapeutic efficacy. This phase 1 study was registered at www.clinicaltrials.gov as #NCT01221571.
Introduction
The majority of Hodgkin lymphoma (HL) patients can be cured with risk-adapted treatment, including chemotherapy and radiotherapy.1 Even when initially diagnosed with advanced-stage disease, >70% of these patients achieve long-term remission.2 However, depending on initial risk factors and treatment, 10% to 30% progress or relapse. Of these patients, only up to 50% can be cured with high-dose chemotherapy and autologous stem cell transplantation (ASCT).3,4 The median overall survival after ASCT failure is ∼2 years.5,6 A significantly poorer outcome was observed for patients with primary progressive HL or relapse within 12 months after initial therapy.7,8 Severe life-threatening treatment-related side effects such as organ toxicity or secondary malignancies might occur during first-line therapy or after treatment.1,9,10
Several new drugs are currently in clinical development for the treatment of relapsed/refractory HL, including small molecules affecting signaling pathways and specific as well as nonspecific immunotherapeutic approaches.11,12 Brentuximab vedotin, an antibody drug conjugate targeting CD30, was the first targeted therapy approved in 2011. Today, brentuximab vedotin is an established treatment of relapsed or refractory HL.13,14 However, although the vast majority of patients respond to this treatment, the median progression-free survival is <6 months.15 This indicates a continuing high medical need for the respective patient population.
Immunotherapies play an increasingly important role in the treatment of hematologic malignancies, including HL. Three immunologic approaches are currently the focus of clinical development in HL: (1) the so-called checkpoint inhibition (eg, nivolumab,16 pembrolizumab,17 and ipilimumab [https://clinicaltrials.gov/ct2/show/NCT01592370]), (2) the modulation of the immune status and tumor environment (eg, lenalidomide18,19 ), or (3) the direct engagement of cytotoxic immune effector cells, such as T cells or natural killer (NK) cells, to mediate tumor cell lysis (eg, by engineering T cells with chimeric antigen receptors, or CAR-T cells [https://clinicaltrials.gov/ct2/show/NCT01192464]) or by recruiting NK cells using bispecific antibodies (AFM13). T cells and NK cells are immunologic effector cells with the potential to fight cancer via tumor cell lysis. About 15 years ago, 2 bispecific antibodies targeting CD30+ tumor cells were investigated in phase 1 clinical studies in HL: an anti-CD30×CD16 and an anti-CD30×CD64 antibody.20,21 Both antibodies showed encouraging signs of clinical activity, but further development was halted due to manufacturing issues.
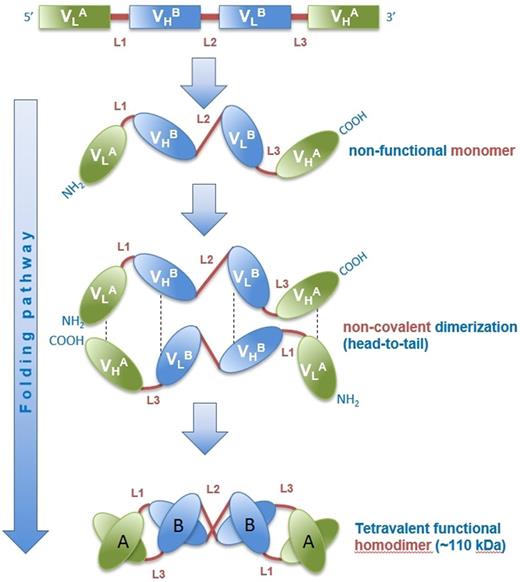
The bispecific, tetravalent chimeric antibody construct (TandAb) AFM13 is the first antibody that specifically recruits NK cells by binding exclusively to the isoform CD16A. TandAbs have 2 binding sites for each antigen but no Fc domains (Figure 1).22,23 AFM13 has a molecular weight of 104 kDa and is produced in mammalian cells. It specifically targets CD30 on HL cells and recruits and activates NK cells by binding to CD16A. Preclinical data demonstrate a specific and efficient antitumor activity via the engagement of NK cells.
Folding pathway of AFM13. The fully functional TandAb antibody is formed by homodimerization of a single polypeptide in a head-to-tail fashion through noncovalent interactions of the immunoglobulin heavy (VH) and light (VL) variable chains of the constituting domains. The human anti-CD16A (FcγRIIIA) antibody domain (VHA/VLA) with specificity for the A isoform of FcγRIII on NK cells and macrophages was isolated from Affimed’s human antibody library. The murine anti-CD30 variable domain (VHB/VLB) was derived from hybridoma HRS-3.
Folding pathway of AFM13. The fully functional TandAb antibody is formed by homodimerization of a single polypeptide in a head-to-tail fashion through noncovalent interactions of the immunoglobulin heavy (VH) and light (VL) variable chains of the constituting domains. The human anti-CD16A (FcγRIIIA) antibody domain (VHA/VLA) with specificity for the A isoform of FcγRIII on NK cells and macrophages was isolated from Affimed’s human antibody library. The murine anti-CD30 variable domain (VHB/VLB) was derived from hybridoma HRS-3.
Here, we present the results of the AFM13-101 “first in humans” phase 1 study in patients with relapsed or refractory HL.
Methods
Patients
The phase 1 clinical study AFM13-101 was conducted at 2 German sites (University Hospital of Cologne and University Medical Center Wuerzburg) and 1 US site (University of Texas, MD Anderson Cancer Center) from September 2010 to December 2012. The study was approved by the relevant institutional review boards or ethics committees, and all patients gave their written informed consent. Patients who relapsed or were refractory after at least 2 prior therapies were eligible. Patients with progressive disease after first-line therapy who were ineligible for high-dose chemotherapy and/or ASCT or any other established curative therapy were also eligible. Further inclusion criteria were age ≥18 years, Eastern Cooperative Oncology Group status ≤2, and signed informed consent. Patients were excluded from the study in case of central nervous system involvement, prior allogeneic stem cell transplantation, any significant disease other than HL, ongoing systemic corticosteroid treatment, known HIV or hepatitis B or C virus infection, or a positive Coombs test result.
Study design and procedures
AFM13-101 was an open-label, single-arm, phase 1 dose-escalation study for patients with relapsed or refractory HL (http://www.clinicaltrials.gov, #NCT01221571). The primary objectives of this study were to evaluate safety and tolerability of AFM13 and to identify the maximum tolerated dose (MTD; the highest dose level at which <33% of patients experience dose-limiting toxicity [DLT]) or the optimum biological dose (OBD; the dose level immediately above the one at which either complete responses and/or partial responses [PR] were observed in 3 patients). Secondary objectives were pharmacokinetics (PK), antitumor activity, and pharmacodynamics of AFM13. The latter included immunologic markers (eg, NK-cell activation), serum outcome markers, cytokine release, and others measured in peripheral blood.
AFM13 was infused once a week for 4 weeks (=1 cycle). This dosing regimen was solely based on in vitro pharmacodynamics and nonclinical PK data in Cynomolgus monkeys, because the establishment of a humanized disease model of HL was not successful.
The first infusion of AFM13 had to be given over 4 hours and was then, if well tolerated, reduced stepwise by 30 minutes up to a minimum duration of 2 hours. The dose-escalation steps were 0.01, 0.04, 0.15, 0.5, 1.5, 4.5, and 7.0 mg/kg body weight. Based on release testings and specifications of drug substance and drug product and in agreement with the Food and Drug Administration, the maximum dose was defined as 7 mg/kg body weight. No premedication was administered. Patients were treated in cohorts of 3 patients per dose level. The number of patients was expanded to 6 in those cohorts in which 1 out of 3 patients developed a DLT, which was defined as any grade 3 or higher toxicity or any treatment delay ≥21 days due to drug-related adverse events (AEs). If none of the 3 patients within a cohort developed a DLT, the dose was escalated. If at least 2 of 6 patients within a cohort experienced a DLT, the dose immediately below this dose level was considered the MTD. Those patients who showed stable disease (SD) or partial or complete response after the first cycle of treatment were eligible to receive 1 additional cycle of AFM13 at the discretion of the investigator. According to the study protocol, an alternative dosing schedule with AFM13 administered twice a week had to be introduced in case the half-life of AFM13 was shorter than 4 days.
Study assessments
Safety was assessed by Common Terminology Criteria for Adverse Events (CTCAE) version 4.02 and included clinical examinations, the assessment of AEs, DLT, and laboratory parameters.
Serum concentrations for AFM13 were measured using an electrochemiluminescence (ECL) assay. Blood samples were collected prior to and immediately after the end of each infusion during cycle 1 in all patients. Furthermore, after the first infusion, blood samples were taken at different time points during and after the infusion. In patients receiving a twice-weekly regimen, an additional sampling identical to the one after the first application was done on the last infusion day. Pharmacokinetic parameters were derived by noncompartmental analyses.
Tumor response was assessed by investigators according to the revised response criteria for malignant lymphoma (Cheson criteria, 2007)24 3 weeks after the last dose of AFM13. Thus, a positron emission tomography–computed tomography scan was mandatory to evaluate the response. Patients were monitored for a minimum of 30 days after the last dose of AFM13. Treatment was to be discontinued upon disease progression. Time to next treatment (TTNT), which is defined as the time between AFM13 treatment and the start of the next treatment, was assessed retrospectively for those patients with PR and SD.
Several immunologic markers in peripheral blood were assessed by a central laboratory. Flow cytometric analysis of NK-cell populations (CD56/16, CD69, CD25, natural cytotoxic receptors, CD71, NKG2D, and CD244), assessment of antibody-dependent cell-mediated cytotoxicity (ADCC) (granzyme B) by enzyme-linked immunosorbent assay, and assessment of complement activation (C3d, CH50) were performed prior to and 12 and 24 hours after the first infusion. Cytokines (interferon-γ, tumor necrosis factor α, interleukin-2 [IL-2], IL-6, IL-10, and IL-12) were measured by Meso Scale Discovery ECL technology prior to, directly after, and 4 and 24 hours after the first infusion. Serum outcome markers (TARC, BAT3, and sMICA) and soluble CD30 (sCD30) were measured prior to and 24 hours after first and last infusion (day 22).
Antidrug antibodies (ADAs) were measured by enzyme-linked immunosorbent assay at baseline and prior to the third, fourth, and last dose. A detection assay using Meso Scale Discovery ECL technology was followed by a competition assay (confirmatory assay). Patient sera testing positive in both assays were finally subjected to an assessment for their neutralizing potential, which was performed by an ADCC assay involving primary human CD16+ NK cells. ADAs were considered neutralizing if the EC50 of AFM13 was increased (neutralizing cut point) in the presence of the patient’s serum compared with its absence.
Data evaluation
An independent data monitoring committee (IDMC) was responsible for the review of safety data on an ongoing basis. A safety review committee comprising the principal investigators, the IDMC, and the medical monitor of the sponsor (Affimed) determined the dose escalation for each step. No formal statistical analyses were performed on safety data. Pharmacokinetic parameters were estimated for each patient using WinNonlin Pro version 5.2.1. A nonlinear power model was used to assess dose proportionality. Tumor assessments and immunologic markers were analyzed by descriptive statistics.
Results
Patients
A total of 28 patients were treated at 3 sites between September 2010 and December 2012. The patients’ characteristics are shown in Table 1. Their median age was 38.5 years (range, 19-72), and the majority were male (57.1%). All patients had classical HL with nodular sclerosis as the most frequent histologic subtype. Although only 9 patients had stage III/IV disease at first diagnosis, 18 patients had stage III/IV disease at initiation of AFM13 treatment. The median time between first diagnosis and initiation of AFM13 treatment was 52 months. The median number of prior therapy regimens was 6 (range, 3-11). A total of 22 patients had been treated with high-dose chemotherapy and ASCT and 24 patients with radiotherapy. Nine patients had a history of brentuximab vedotin treatment; 7 of them received brentuximab vedotin as most recent treatment prior to AFM13. A total of 14 patients were refractory, and 14 patients had relapsed after their most recent therapy.
Patient characteristics
| . | Value . |
|---|---|
| Age (y), median (range) | 38.5 (19-72) |
| Male | 16 (57.1) |
| Diagnosis CD30+ classical HL | 28 (100) |
| Previous treatments | |
| Stages III/IV at first diagnosis | 9 (32.1) |
| Months between first diagnosis and AFM13 initiation, median (range) | 52 (8-468) |
| Previous treatment lines, median (range) | 6 (3-11) |
| Previous radiotherapy | 24 (85.7) |
| Previous ASCT | 22 (78.6) |
| Previous brentuximab vedotin | |
| Total | 9 (28.6) |
| As most recent therapy | 7 (25.0) |
| Disease status at initiation of AFM13 | |
| Stages III/IV | 18 (64.3) |
| B-symptoms | 13 (46.5) |
| Extranodal manifestation | 12 (42.9) |
| Large mediastinal tumor | 3 (10.7) |
| ≥3 LN regions | 18 (64.3) |
| LDH >240 U/L | 15 (53.6) |
| ECOG status 0 | 12 (42.9) |
| ECOG status 1 | 16 (57.1) |
| ECOG status 2 | 0 (0.0) |
| . | Value . |
|---|---|
| Age (y), median (range) | 38.5 (19-72) |
| Male | 16 (57.1) |
| Diagnosis CD30+ classical HL | 28 (100) |
| Previous treatments | |
| Stages III/IV at first diagnosis | 9 (32.1) |
| Months between first diagnosis and AFM13 initiation, median (range) | 52 (8-468) |
| Previous treatment lines, median (range) | 6 (3-11) |
| Previous radiotherapy | 24 (85.7) |
| Previous ASCT | 22 (78.6) |
| Previous brentuximab vedotin | |
| Total | 9 (28.6) |
| As most recent therapy | 7 (25.0) |
| Disease status at initiation of AFM13 | |
| Stages III/IV | 18 (64.3) |
| B-symptoms | 13 (46.5) |
| Extranodal manifestation | 12 (42.9) |
| Large mediastinal tumor | 3 (10.7) |
| ≥3 LN regions | 18 (64.3) |
| LDH >240 U/L | 15 (53.6) |
| ECOG status 0 | 12 (42.9) |
| ECOG status 1 | 16 (57.1) |
| ECOG status 2 | 0 (0.0) |
Data are n (%) unless otherwise indicated. ECOG, Eastern Cooperative Oncology Group; LDH, lactate dehydrogenase; LN, lymph node.
In each of the dose cohorts of the weekly AFM13 regimen, 3 patients were treated, with the exception of cohort 4 (0.5 mg/kg), in which 6 patients were treated due to the occurrence of a DLT. A total of 24 patients completed the dose-escalation phase of the weekly AFM13 regimen with a maximum dose of 7 mg/kg body weight according to the study protocol. Five of these 24 patients received a second cycle of therapy: 2 in cohort 3 (0.15 mg/kg) and 1 in each of cohorts 4 (0.5 mg/kg), 5 (1.5 mg/kg), and 6 (4.5 mg/kg). Four additional patients were treated with a twice-weekly regimen of 4.5 mg/kg over 4 weeks.
Safety
All 28 patients received at least 1 infusion of AFM13 and were included in the safety population. All patients had a minimum of 4 infusions (1 cycle), except for 1 patient in cohort 4 (0.5 mg/kg) who received only 3 weekly infusions. This patient was the only one who discontinued treatment due to the occurrence of a serious AE, which was also the only DLT observed in the study (see below).
Upon the completion of the per-protocol dose-escalation phase, the MTD was not reached. A total of 27 of 28 patients developed at least 1 AE, and most AEs were mild to moderate. The most common AEs occurring in ≥4 patients were fever (15 patients, 53.6%), chills (11 patients, 39.3%), headache (8 patients, 28.6%), nausea and nasopharyngitis (5 patients each, 17.9%), and infusion reaction, rash, vomiting, and pneumonia (4 patients each, 14.3%) (Table 2). Of the above-mentioned most common events, only 1 case of fever and 4 cases of pneumonia were CTCAE grade ≥3. Only 1 of the 4 pneumonia cases was considered to be possibly related to treatment. Overall, 3 patients with pneumonia, including the possibly related case, recovered after initiation of antibiotic treatment, and 1 patient developed a fungal pneumonia as described below.
Patients with the most frequent AEs (occurring in 4 or more patients) by preferred terms (safety population)
| Preferred term . | Safety population (n = 28) . | CTCAE grade 1/2 . | CTCAE grade ≥3 . |
|---|---|---|---|
| Pyrexia | 15 (53.6) | 14 (50.0) | 1 (3.6) |
| Chills | 11 (39.3) | 11 (39.3) | 0 (0.0) |
| Headache | 8 (28.6) | 8 (28.6) | 0 (0.0) |
| Nausea | 5 (17.9) | 5 (17.9) | 0 (0.0) |
| Nasopharyngitis | 5 (17.9) | 5 (17.9) | 0 (0.0) |
| Vomiting | 4 (14.3) | 4 (14.3) | 0 (0.0) |
| Pneumonia | 4 (14.3) | 0 (0.0) | 4 (14.3) |
| Infusion reaction | 4 (14.3) | 4 (14.3) | 0 (0.0) |
| Rash | 4 (14.3) | 4 (14.3) | 0 (0.0) |
| Preferred term . | Safety population (n = 28) . | CTCAE grade 1/2 . | CTCAE grade ≥3 . |
|---|---|---|---|
| Pyrexia | 15 (53.6) | 14 (50.0) | 1 (3.6) |
| Chills | 11 (39.3) | 11 (39.3) | 0 (0.0) |
| Headache | 8 (28.6) | 8 (28.6) | 0 (0.0) |
| Nausea | 5 (17.9) | 5 (17.9) | 0 (0.0) |
| Nasopharyngitis | 5 (17.9) | 5 (17.9) | 0 (0.0) |
| Vomiting | 4 (14.3) | 4 (14.3) | 0 (0.0) |
| Pneumonia | 4 (14.3) | 0 (0.0) | 4 (14.3) |
| Infusion reaction | 4 (14.3) | 4 (14.3) | 0 (0.0) |
| Rash | 4 (14.3) | 4 (14.3) | 0 (0.0) |
Data are presented as n (%) of patients.
A total of 9.2% of all observed AEs were grade ≥3, with 8 patients (28.6%) experiencing at least 1 AE of grade ≥3 (Table 3). A total of 51.8% of the events were considered to be treatment related, of which almost all occurred during or shortly after the AFM13 administration and were evaluated as infusion-related reactions.
AEs of CTCAE grade 3 or higher (safety population)
| System organ class and preferred term . | Cohort 1: 0.01 mg/kg (n = 3) . | Cohort 2: 0.04 mg/kg (n = 3) . | Cohort 3: 0.15 mg/kg (n = 3) . | Cohort 4: 0.5 mg/kg (n = 6) . | Cohort 5: 1.5 mg/kg (n = 3) . | Cohort 6: 4.5 mg/kg (n = 3) . | Cohort 7: 7 mg/kg (n = 3) . | Cohort 8: 2 × 4.5 mg/kg (n = 4) . | Overall (n = 28) . |
|---|---|---|---|---|---|---|---|---|---|
| Any AE of CTCAE grade ≥3 | 0 | 0 | 0 | 1 (16.7) | 3 (100.0) | 1 (33.3) | 2 (66.7) | 1 (25.0) | 8 (28.6) |
| Blood and lymphatic disorders | |||||||||
| Anemia | 0 | 0 | 0 | 1 (16.7) | 0 | 1 (33.3) | 0 | 0 | 2 (7.1) |
| Hemolytic anemia | 0 | 0 | 0 | 1 (16.7) | 0 | 0 | 0 | 0 | 1 (3.6) |
| Thrombocytopenia | 0 | 0 | 0 | 1 (16.7) | 0 | 0 | 0 | 0 | 1 (3.6) |
| General disorders | |||||||||
| Multiorgan failure | 0 | 0 | 0 | 1 (16.7) | 0 | 0 | 0 | 0 | 1 (3.6) |
| Pyrexia | 0 | 0 | 0 | 0 | 1 (33.3) | 0 | 0 | 0 | 1 (3.6) |
| Thrombosis in device | 0 | 0 | 0 | 0 | 1 (33.3) | 0 | 0 | 0 | 1 (3.6) |
| Infections and infestations | |||||||||
| Bronchitis | 0 | 0 | 0 | 0 | 1 (33.3) | 0 | 0 | 0 | 1 (3.6) |
| Pneumonia | 0 | 0 | 0 | 1 (16.7) | 1 (33.3) | 0 | 1 (33.3) | 1 (25.0) | 4 (14.3) |
| Staphylococcal infection | 0 | 0 | 0 | 0 | 0 | 0 | 1 (33.3) | 0 | 1 (3.6) |
| Investigations | |||||||||
| Bilirubin increased | 0 | 0 | 0 | 1 (16.7) | 0 | 0 | 0 | 0 | 1 (3.6) |
| Metabolism and nutrition | |||||||||
| Hypoalbuminemia | 0 | 0 | 0 | 1 (16.7) | 0 | 0 | 0 | 0 | 1 (3.6) |
| Neoplasms | |||||||||
| T-cell lymphoma | 0 | 0 | 0 | 0 | 1 (33.3) | 0 | 0 | 0 | 1 (3.6) |
| System organ class and preferred term . | Cohort 1: 0.01 mg/kg (n = 3) . | Cohort 2: 0.04 mg/kg (n = 3) . | Cohort 3: 0.15 mg/kg (n = 3) . | Cohort 4: 0.5 mg/kg (n = 6) . | Cohort 5: 1.5 mg/kg (n = 3) . | Cohort 6: 4.5 mg/kg (n = 3) . | Cohort 7: 7 mg/kg (n = 3) . | Cohort 8: 2 × 4.5 mg/kg (n = 4) . | Overall (n = 28) . |
|---|---|---|---|---|---|---|---|---|---|
| Any AE of CTCAE grade ≥3 | 0 | 0 | 0 | 1 (16.7) | 3 (100.0) | 1 (33.3) | 2 (66.7) | 1 (25.0) | 8 (28.6) |
| Blood and lymphatic disorders | |||||||||
| Anemia | 0 | 0 | 0 | 1 (16.7) | 0 | 1 (33.3) | 0 | 0 | 2 (7.1) |
| Hemolytic anemia | 0 | 0 | 0 | 1 (16.7) | 0 | 0 | 0 | 0 | 1 (3.6) |
| Thrombocytopenia | 0 | 0 | 0 | 1 (16.7) | 0 | 0 | 0 | 0 | 1 (3.6) |
| General disorders | |||||||||
| Multiorgan failure | 0 | 0 | 0 | 1 (16.7) | 0 | 0 | 0 | 0 | 1 (3.6) |
| Pyrexia | 0 | 0 | 0 | 0 | 1 (33.3) | 0 | 0 | 0 | 1 (3.6) |
| Thrombosis in device | 0 | 0 | 0 | 0 | 1 (33.3) | 0 | 0 | 0 | 1 (3.6) |
| Infections and infestations | |||||||||
| Bronchitis | 0 | 0 | 0 | 0 | 1 (33.3) | 0 | 0 | 0 | 1 (3.6) |
| Pneumonia | 0 | 0 | 0 | 1 (16.7) | 1 (33.3) | 0 | 1 (33.3) | 1 (25.0) | 4 (14.3) |
| Staphylococcal infection | 0 | 0 | 0 | 0 | 0 | 0 | 1 (33.3) | 0 | 1 (3.6) |
| Investigations | |||||||||
| Bilirubin increased | 0 | 0 | 0 | 1 (16.7) | 0 | 0 | 0 | 0 | 1 (3.6) |
| Metabolism and nutrition | |||||||||
| Hypoalbuminemia | 0 | 0 | 0 | 1 (16.7) | 0 | 0 | 0 | 0 | 1 (3.6) |
| Neoplasms | |||||||||
| T-cell lymphoma | 0 | 0 | 0 | 0 | 1 (33.3) | 0 | 0 | 0 | 1 (3.6) |
Data are presented as n (%) of AEs.
The only DLT observed in the study was a hemolytic anemia (CTCAE grade 4) in a patient treated in cohort 4 (0.5 mg/kg) after he received 3 infusions of AFM13, which the investigator considered to be possibly related to the treatment. This DLT could not be followed up further because the patient subsequently developed a fungal pneumonia (probably by Aspergillus) with sepsis and fatal multiorgan failure. These events were considered unlikely to be related to the study drug. No further relevant anemia was observed during the course of the study. There was 1 further death; a patient treated in cohort 5 (1.5 mg/kg), who received all 4 infusions, died of a progressive pulmonary infiltrate of HL, which was histologically confirmed and considered unlikely to be related to the study drug.
Overall, neither the number nor the severity or relatedness of reported AEs increased during the dose escalation from 0.5 mg/kg up to the highest dose of 7 mg/kg body weight. Also, a higher dose density in the twice-weekly regimen of 4.5 mg/kg did not result in a different safety profile.
ADAs were detected in 15 out of 28 patients during the course of treatment and were present in all dose cohorts except for the 4.5-mg/kg twice-weekly cohort. Of note, 4 patients had detectable ADA only at 1 time point prior to or early during the treatment period (cycle 1, day 1 or 15) and never again thereafter. In 8 out of 15 patients, ADAs with neutralizing potency could be detected (1 patient in the 0.4-mg/kg cohort, 2 patients in the 0.15-mg/kg cohort, 2 patients in the 1.5-mg/kg cohort, and 3 patients in the 0.5-mg/kg cohort). Sera revealing the highest neutralizing potential were derived from a patient treated with 0.15 mg/kg followed by a patient in the 1.5-mg/kg cohort. In all other patients, the neutralizing potential was low (ie, just above the defined neutralizing cut point). It is not known against which immunogenic structure of AFM13 ADAs were directed.
Pharmacokinetics
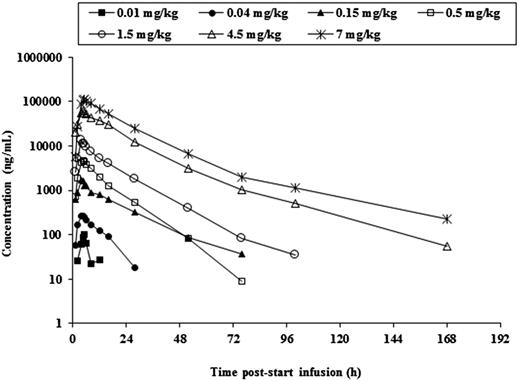
Systemic exposure of AFM13 increased with escalating doses in a manner slightly greater than dose proportional. Figure 2 shows the mean AFM13 serum concentration following a single infusion of different doses of AFM13. The mean terminal half-life for the different dose cohorts was in the range of 8.72 to 19.2 hours, with longer half-life at higher doses. The kinetics of AFM13 appeared to be time invariant. The distribution volume was in the range of 60.7 to 125 mL/kg, which is not reasonably different from blood volume.
Mean serum concentrations of AFM13 following a single IV infusion of increasing doses of AFM13.
Mean serum concentrations of AFM13 following a single IV infusion of increasing doses of AFM13.
Response to treatment
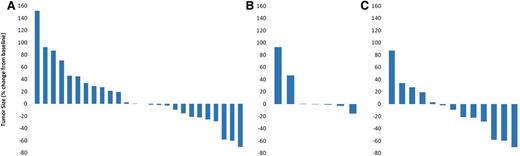
A total of 26 of 28 patients were eligible for efficacy evaluation. In 2 patients, tumor response data were missing: 1 patient (cohort 1; 0.01 mg/kg) received all 4 infusions but left the study before tumor assessment could be done, and a second patient (cohort 4; 0.5 mg/kg) received only 3 infusions when study participation was discontinued due to AEs. The overall response rate (ORR) was 11.5%, with 3 patients achieving PR and 13 patients (50%) achieving SD, resulting in a disease control rate of 61.5%. Ten patients (38.5%) had progressive disease (PD) (Table 4 and Figure 3A). Partial responses were observed in 2 patients in the 1.5-mg/kg weekly cohort and 1 patient in the 4.5-mg/kg twice-weekly cohort. Thus, the OBD was not identified. Of the 9 patients previously treated with brentuximab vedotin, 7 had received brentuximab vedotin as the most recent treatment. In all 7 patients, treatment was discontinued because of PD. Six out of 7 patients achieved a SD through treatment with AFM13. Figure 3B shows a waterfall plot with the respective relative changes in tumor volume for these patients during AFM13 treatment.
Response summary
| Best response to AFM13, efficacy population (n = 26) . | Patients, n (%) . |
|---|---|
| Complete remission | 0 (0.0) |
| Partial response | 3 (11.5) |
| Stable disease | 13 (50.0) |
| Progressive disease | 10 (38.5) |
| Disease control rate | 61.5 |
| Best response to AFM13, efficacy population (n = 26) . | Patients, n (%) . |
|---|---|
| Complete remission | 0 (0.0) |
| Partial response | 3 (11.5) |
| Stable disease | 13 (50.0) |
| Progressive disease | 10 (38.5) |
| Disease control rate | 61.5 |
Change in the sum of the product of diameters, measured by computed tomography. (A) Efficacy population (n = 26). (B) Patients refractory to brentuximab vedotin as the most recent treatment prior to AFM13 (n = 7). (C) Patients treated with AFM doses ≥1.5 mg/kg body weight (n = 13).
Change in the sum of the product of diameters, measured by computed tomography. (A) Efficacy population (n = 26). (B) Patients refractory to brentuximab vedotin as the most recent treatment prior to AFM13 (n = 7). (C) Patients treated with AFM doses ≥1.5 mg/kg body weight (n = 13).
Because patients were not followed up further after treatment, no data on progression-free survival or duration of response are available. However, TTNT was assessed retrospectively for those patients with PR and SD. TTNT was in the range of 1.5 to 9 months, with a mean of 5.1 months and a median of 5 months.
Biomarker analysis
AFM13 treatment resulted in a decrease of the total number of detectable circulating NK cells immediately after infusion. This effect was transient, and total NK-cell numbers were back to baseline levels prior to the next infusion (data not shown). In parallel, the relative portion of activated NK cells, indicated by CD69+, increased immediately after the infusions, as reported previously by Reiners et al.25 This observation was dose independent and strongest after the first infusion. However, during the period between the infusions, activated NK cells decreased again to baseline levels prior to the next infusion. Figure 4 shows the relative number of activated NK cells (CD69+) measured in peripheral blood in all patients treated with ≥0.15 mg/kg AFM13 (n = 22). No sufficient data are available for patients receiving 0.01 and 0.04 mg/kg AFM13.
Number of activated NK cells (CD69+) relative to total number of NK cells (CD16+ or CD56+; CD3−). Change from baseline (=100%) for all patients receiving doses ≥0.15 mg/kg AFM13 (n = 22).
Number of activated NK cells (CD69+) relative to total number of NK cells (CD16+ or CD56+; CD3−). Change from baseline (=100%) for all patients receiving doses ≥0.15 mg/kg AFM13 (n = 22).
AFM13 had a significant, dose-dependent effect on sCD30 levels in serum of patients. Although sCD30 levels decreased on average by 27% in patients receiving doses <1.5 mg/kg AFM13, levels were decreased by 89% in patients receiving doses of ≥1.5 mg/kg.
Quantifiable serum cytokine levels could only be measured for IL-6 (n = 8), IL-8 (n = 4), IL-10 (n = 3), and tumor necrosis factor α (n = 7). No cytokine release was detected in patients receiving doses <0.5 mg/kg AFM13. Data on cytokine release were inconclusive regarding a correlation with dose or activity of AFM13. Similarly, assessment of ADCC activity through quantification of granzyme B and serum outcome markers TARC, BAT3, and sMICA did not provide conclusive information. Although for most of these parameters, the serum concentrations were below the detection limit, only TARC could be quantified in the majority of patients. However, TARC levels varied from patient to patient and did not show any relationship to the AFM13 dose administered or clinical effect observed (data not shown).
Discussion
Antibody-mediated recruitment of cytotoxic immune effector cells to tumors using bispecific antibodies is a cell-based immunotherapeutic approach for the treatment of hematologic malignancies. Blinatumomab, a CD19×CD3 bispecific T-cell engager (BiTE; Amgen), has shown impressive efficacy in patients with acute lymphoblastic leukemia and diffuse large B-cell lymphoma.26,27 AFM13 is a novel NK-cell–recruiting antibody that targets CD30 and CD16A and that may provide a new treatment option for patients with relapsed or refractory HL. As compared with T cells, which belong to the adaptive immune system, NK cells are part of the innate immune system with the potential to recognize and destroy degenerated and neoplastic cells.
In the phase 1 study reported here, AFM13 was investigated in heavily pretreated HL patients who had received all standard therapies. Although preclinical in vitro data indicated the potency and specificity of AFM13 to kill CD30+ cells,23 there was no appropriate in vivo model to demonstrate safety and efficacy of NK-cell activation by AFM13 against HL cells. In addition, there was no experience with antibodies specifically targeting CD16A. Therefore, in agreement with competent authorities, AFM13 dose and regimen were selected with the focus on the patient’s safety rather than for showing efficacy. The dosing started very low and was then escalated 700-fold. Further, a low dose intensity was selected with weekly doses over 4 weeks per cycle. If AFM13 proved to be safe during the escalation and the PK data indicated that a more frequent dosing is reasonable, a twice-weekly regimen could be investigated.
The treatment with AFM13 was well tolerated, with dominantly mild to moderate AEs. Fever and chills were the most frequent events, all of which were managed through standard supportive care, without the need for premedication. One patient in the 0.5-mg/kg dose cohort developed a possibly drug-induced grade 4 hemolytic anemia. This patient died of an invasive fungal disease before completion of the study that was not related to AFM13. Referring to preclinical data, there has been no indication for an increased risk of hemolytic anemia. However, autoimmune hemolytic anemia was described in HL patients, in particular in stages III and IV of the disease.28 No further signs of hemolytic anemia were observed in other patients treated with AFM13, even at doses that were up to 14 times higher. Overall, the MTD was not reached in the study, and an IDMC indicated no safety concerns for the further development of AFM13.
Although the MTD was not reached, this phase 1 study demonstrated an acceptable safety profile of AFM13. The safety profile was stable for AFM13 doses in the range from 0.05 mg/kg to 7 mg/kg (ie, during a 140-fold dose increase). Also, a more dose-intense, twice-weekly regimen of 4.5 mg/kg did not result in a higher risk for the patients. Based on the safety data of this phase 1 trial, it can therefore be concluded that the application of AFM13 in an increased dose intensity in future studies should also be safe and feasible.
The tetravalent bispecific TandAb AFM13 seems to have a favorable PK profile compared with smaller bivalent, bispecific antibodies such as the BiTE antibodies, which have to be administered as a continuous infusion over several weeks.27,29 The longer half-life of AFM13 of up to 19 hours is caused by the molecular weight of 104 kDa, which is double that of the BiTEs and prevents a fast elimination by renal filtration. However, due to the missing Fc-fragment of TandAbs, the half-life is shorter than those of full-length antibodies. Therefore, the dosing should be more frequent than weekly over the first 1 to 2 weeks of treatment in order to maintain a basic trough serum level during the initial saturation phase of treatment.
ADAs were detected in about half of the patients. It is not known against which part of AFM13 the antibodies are directed. Of note, AFM13 is a chimeric antibody with a murine anti-CD30 variable domain. Half of the detected ADAs had neutralizing potential. However, no impact of ADAs on safety or efficacy could be shown in this small study. As expected, there was no correlation of ADA development with the dose administered. Since PK was only measured after the first infusion, the impact of neutralizing ADAs on PK parameters could not be assessed. These facts, together with the small sample size of this phase 1 study, warrant further investigations of the development of ADAs in future clinical studies.
Because there was no study cohort with 3 responders, the OBD could not be identified. However, the data of this phase 1 study indicate that clinical and pharmacodynamic activity of AFM13 was more pronounced at doses of ≥1.5 mg/kg. Looking at data of respective dose cohorts, ie, ≥1.5 mg/kg (n = 13), the ORR was 23% and the disease control rate was 77%. All patients had progressive disease at AFM13 initiation, and tumor shrinkage was observed in 8 of 13 patients (61.5%) treated with AFM13 (Figure 3C).
Importantly, tumor shrinkage was also observed in 3 out of 7 patients refractory to their most recent treatment with brentuximab vedotin, and only 1 out of 7 patients had PD. Like AFM13, brentuximab vedotin targets CD30; however, the effector mechanism of these substances is entirely different: AFM13 activates the patient’s cell-based immune system to target the tumor, whereas brentuximab vedotin delivers a chemotherapeutic agent into the lymphoma cell. Chemotherapies usually result in rapid clinical effects; however, the safety profile is often less favorable, and resistance to the cytotoxic components occurs frequently in relapsed or refractory settings. These characteristics have also been observed with brentuximab vedotin: the ORR for brentuximab vedotin was 50% in a phase 1 study in relapsed or refractory HL30 and 75% in the registration phase 2 study.15 On the other hand, the duration of the effect was short with an overall progression-free survival of <6 months, and the safety was less favorable, with peripheral neuropathy occurring in 42% of the patients in the phase 2 study.15 In contrast to that, clinical response to immunotherapies may occur late, but it may be of longer duration.31
One hurdle for the development of cancer immunotherapies is the so-called pseudoprogression, which can lead physicians to discontinue treatment even though the tumor did not truly progress.32 In fact, physicians who treated patients in this clinical study described a “flare-up” of the tumor lesions in at least 2 patients shortly after starting AFM13 treatment. It has therefore to be further investigated whether this flare is caused by tumor growth or by infiltrating immune cells. Consequently, tumor assessments should not be done too early because of potentially misleading results. In addition, a 4-week therapy with AFM13 is most likely not sufficient to reach the maximum therapeutic effect of the immunotherapy.
The biomarker analyses showed that AFM13 treatment resulted in a decrease of total NK-cell numbers in the peripheral blood immediately after infusion, which resolved completely during the treatment interval. We assume that this was caused by recruitment of NK cells to tissue/endothelia rather than depletion. Furthermore, AFM13 induced a clear activation of NK cells measured in peripheral blood. The kinetics of NK-cell activation were close to the PK; ie, after a peak following AFM13 infusion, values decreased to baseline prior to the next infusion (Figures 2 and 4). These results of NK-cell biomarker analyses strongly suggest that a weekly regimen, in particular over the first days or weeks of treatment when a high antigen load is available, may not be sufficient for saturation. This underlines the need for a modified dose regimen of AFM13, at least over the first treatment period, and is consistent with conclusions from PK findings.
The concentration of sCD30 clearly decreased after the administration of AFM13. It is currently uncertain whether this effect is due to binding of AFM13 to sCD30 or due to the antitumor effect of AFM13. Further biomarkers deserve more investigation because of a lack of conclusive data from this phase 1 study. However, it is well known that markers measured in peripheral blood do not necessarily represent the situation in the tumor or its environment. It is therefore of utmost importance to take biopsy specimens prior to and during AFM13 treatment to better understand the immunologic process.
Although H/RS cells are surrounded by a prominent lymphocytic infiltration,33 HL is characterized by its unique ability to cause immunodeficiency in terms of an antilymphoma immune response, as well as to provide immune-evasion mechanisms.34,35 Poppema et al have demonstrated that specific cytotoxic T- or NK-cell populations are absent in the environment of H/RS cells.36 Reiners et al25 investigated functional NK-cell defects and found that in peripheral blood of HL patients, NK-cell function is impaired. This impairment correlated with the downregulation of the NK-cell receptors NKp30 and, in particular, NKG2D. Consequently, using a CD30+ cell line (L428), the authors could demonstrate in vitro that the cytotoxic activity of NK cells isolated from blood of HL patients was significantly reduced compared with NK cells from healthy donors. They further demonstrated that the addition of AFM13 to NK cells from HL patients resulted in a restoration of cytotoxicity. Furthermore, in the framework of this phase 1 study, ex vivo killing assays with isolated NK cells from patients were also performed by the authors. Although NK cells before therapy were inactive, NK cells isolated after AFM13 treatment showed a cytotoxic activity close to NK cells from healthy donors. Thus, AFM13 could overcome immune-escape mechanisms of HL.
In conclusion, AFM13 was well tolerated and demonstrated clinical and pharmacodynamic activity in this phase 1 study. AFM13 represents a new, feasible, targeted immunotherapy for heavily pretreated patients with HL. The dose regimen of AFM13 has to be optimized and the treatment duration has to be prolonged in order to increase the clinical efficacy. Biomarkers have to be further investigated, and biopsy specimens should be taken to broaden the knowledge about the NK-cell activity as well as other immunologic processes during AFM13 therapy. A phase 2 study considering these aspects is currently in preparation.
There is an Inside Blood Commentary on this article in this issue.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
This study was supported by Affimed.
Authorship
Contribution: A.R., M.R., and A.E. designed the trial; A.R., S.S., D.A.E., and C.B. coordinated the trial; A.R., S.S., M.S.T., D.A.E., H.H., C.B., and A.E. treated patients; A.R., S.S., M.D., G.K., M.R., S.K., J.-P.M., and A.E. analyzed and interpreted data; A.R., S.S., and J.-P.M. wrote the manuscript; and M.S.T., D.A.E., H.H., K.S.R., M.D., G.K., J.K., C.B., M.R., S.K., E.P.v.S., P.B., and A.E. reviewed the manuscript.
Conflict-of interest disclosure: A.E. received research support and honoraria from Affimed. M.S.T. is a member of the Affimed and Amgen advisory boards, received a research grant and travel support from Affimed, and received travel support from Amgen. K.S.R. and E.P.v.S. received research support from Affimed. M.R. is a former consultant of Affimed. S.K. and J.-P.M. are employees of Affimed. The remaining authors declare no competing financial interests.
Correspondence: Andreas Engert, Department I of Internal Medicine, University Hospital of Cologne, Kerpener Str 62, 50937 Cologne, Germany; e:mail: a.engert@uni-koeln.de.
References
Author notes
A.R. and S.S. contributed equally to this study.