Key Points
PF-04691502 induces potent apoptosis in CLL cells and suppresses prosurvival anti–immunoglobulin M signaling and CXCL12-induced migration.
PF-04691502 displays powerful antitumor effects in vivo in the Eμ-TCL1 mouse model.
Abstract
Current treatment strategies for chronic lymphocytic leukemia (CLL) involve a combination of conventional chemotherapeutics, monoclonal antibodies, and targeted signaling inhibitors. However, CLL remains largely incurable, with drug resistance and treatment relapse a common occurrence, leading to the search for novel treatments. Mechanistic target of rapamycin (mTOR)-specific inhibitors have been previously assessed but their efficacy is limited due to a positive feedback loop via mTOR complex 2 (mTORC2), resulting in activation of prosurvival signaling. In this study, we show that the dual phosphatidylinositol 3-kinase (PI3K)/mTOR inhibitor PF-04691502 does not induce an mTORC2 positive feedback loop similar to other PI3K inhibitors but does induce substantial antitumor effects. PF-04691502 significantly reduced survival coincident with the induction of Noxa and Puma, independently of immunoglobulin heavy chain variable region mutational status, CD38, and ZAP-70 expression. PF-04691502 inhibited both anti–immunoglobulin M–induced signaling and overcame stroma-induced survival signals and migratory stimuli from CXCL12. Equivalent in vitro activity was seen in the Eμ-TCL1 murine model of CLL. In vivo, PF-04691502 treatment of tumor-bearing animals resulted in a transient lymphocytosis, followed by a clear reduction in tumor in the blood, bone marrow, spleen, and lymph nodes. These data indicate that PF-04691502 or other dual PI3K/mTOR inhibitors in development may prove efficacious for the treatment of CLL, increasing our armamentarium to successfully manage this disease.
Introduction
Chronic lymphocytic leukemia (CLL) is characterized by the accumulation of CD5+CD19+ cells in the peripheral blood, lymph nodes, and bone marrow. Several prognostic markers are associated with progressive disease, including unmutated immunoglobulin heavy chain variable regions (IGHV) and high expression of CD38 and ZAP-70.1 Current treatment strategies involve the combination of fludarabine, cyclophosphamide, and rituximab2 ; however, CLL largely remains incurable, with drug resistance and treatment relapse being common occurrences.3
Recently, inhibitors targeting key signaling molecules such as Bruton tyrosine kinase (BTK; ibrutinib) and phosphatidylinositol 3-kinase (PI3K; idelalisib) have been approved4,5 due to exceptional clinical responses.6-11 In this regard, the PI3K/mTOR pathway is particularly attractive because it is activated upon ligation of various chemokine and cytokine receptors expressed by CLL cells, as well as after B cell–receptor (BCR) engagement.12 Class 1 PI3Ks are divided into class 1A (PI3Kα, PI3Kβ, and PI3Kδ) and class 1B (PI3Kγ) isoforms. The expression of PI3Kδ and PI3Kγ is largely restricted to leukocytes, whereas the expression of PI3Kα and PI3Kβ is ubiquitous. PI3Kδ and PI3Kγ have crucial roles in a plethora of leukocyte functions, including proliferation, antibody secretion, survival, migration,13 and reactive oxygen species generation.14 Functional redundancy between PI3K isoforms is evident and supported by the fact that multiple isoforms require inhibition to fully reverse the neutrophil survival induced by granulocyte macrophage–colony-stimulating factor.15 PI3K exists in a complex signaling network with multiple partners, including those regulating mTOR. mTOR complex 1 (mTORC1) is a serine/threonine kinase activated downstream of PI3K that controls protein translation, growth, and proliferation in part via modulation of S6 ribosomal subunit activity.16 In contrast, mTOR complex 2 (mTORC2) phosphorylates AKTS473 and is required for maximal activation of protein kinase B (AKT)16 (supplemental Figure 1A-B, available on the Blood Web site).
PI3K signaling is known to be overactive in CLL,17 with patients with unmutated IGHV having increased PI3K gene expression compared with patients with mutated IGHV.18 The PI3Kδ selective inhibitor idelalisib has been approved by the US Food and Drug Administration to treat CLL patients with relapsed disease in combination with rituximab.4 Idelalisib inhibits chemokine- and BCR-induced signaling and sensitizes CLL cells to standard genotoxic agents.19-21 In patients, idelalisib has a dual mechanism of action: (1) it directly reduces CLL cell viability and (2) it disrupts stromal cell interactions and releases CLL cells from their protective microenvironments into the blood, where they are more susceptible to chemotherapy-induced apoptosis. However, single-isoform inhibitors against PI3Kα/β/δ and pan–class-1 PI3K inhibitors in CLL cells have all been reported to induce apoptosis.22,23 Pharmacologic inhibition of mTOR induces cell cycle arrest and apoptosis in CLL cells24-26 ; however, prolonged inhibition of mTOR is known to disrupt negative feedback loops and cause increased AKTS473 activation in other malignancies.27,28
Given the important role of both PI3K and mTOR in CLL cell survival, the dual pharmacologic inhibition of both class-1 PI3K and mTOR signaling may offer new therapeutic potential, with the possibility of deeper remissions or as an alternative after resistance to idelalisib. PF-04691502 is a potent selective dual class-1 pan-PI3K/mTOR inhibitor29 shown to inhibit tumor growth and to promote apoptosis in solid tumors.29-33 PF-04691502 inhibits PI3Kδ, PI3Kα, PI3Kγ, and PI3Kβ at 50% inhibitory concentration values (IC50) of 1.6 nM (Ki), 1.8 nM (Ki), 1.9 nM (Ki), and 2.1 nM (Ki), respectively. In cells, inhibition with PF-04691502 has resulted in IC50 values of 3.8 nM and 7.5 nM for P-AKTS473 and P-AKTT308, respectively.29 During phase 1 clinical trials, PF-04691502 was shown to have a similar safety profile to other PI3K inhibitors.34
In this study, we showed that in primary CLL cells, PF-04691502 induced caspase-dependent apoptosis. In contrast, little effect on the viability of normal B and T lymphocytes was evident. BCR- and CXCR4-stimulated signaling was inhibited by PF-04691502, and chemokine-mediated motility was potently reduced. Similarly impressive antitumor effects were seen with the Eμ-TCL1 murine CLL-like cells in vitro and in vivo. Together, these data indicate that concomitant targeting of mTOR and PI3K is a powerful approach for treating CLL.
Materials and methods
Patients and cells
Diagnosis of CLL was according to the International Workshop on CLL 2008 criteria.35 Forty-nine CLL isolates were studied after informed written consent and in accordance with ethics committee approvals under the Declaration of Helsinki (reference 228/02/t) (supplemental Table 1). Procedures for the isolation of malignant cells and the determination of their purity have been described previously.36 All isolates contained >90% CD19+CD5+ cells. Normal B and T lymphocytes from peripheral blood were taken from healthy age-matched controls as previously described.37
Reagents
Tissue culture materials were from Life Technologies (Paisley, United Kingdom). ZVAD.fmk was from Enzo Life Sciences (Exeter, United Kingdom). PF-04691502 was provided by Pfizer and purchased from Selleck Chemicals (Houston, TX) for in vivo studies. Idelalisib, BYL719, everolimus, GSK2636771, and AS-605240 were from Selleck Chemicals. Annexin V was from the Southampton Cancer Research United Kingdom core proteomics facility. Anti-IgM was from Cambridge Bioscience (Cambridge, United Kingdom) and CXCL12 was from Miltenyi Biotec (Bisley, United Kingdom). HFFF2 cells were a kind gift from Professor Thomas (University of Southampton).
Cell culture and protein extraction
CLL cell culture and protein extraction for immunoblot analysis were performed as previously described.38,39 Soluble anti-immunoglobulin (Ig)M was used at 20 μg/mL; bead-bound immobilized anti-IgM was added at a 2:1 ratio of beads to CLL cells. Densitometry of immunoblots is depicted in supplemental Figures 6 and 7.
Gel electrophoresis and immunoblotting
Proteins were separated on 12% polyacrylamide gels (Life Technologies), transferred to nitrocellulose membranes (GE Healthcare, Buckinghamshire, United Kingdom), and probed with antibodies listed in supplemental Table 2. Bands were detected by incubation with horseradish peroxidase–linked secondary antibodies (Dako), enhanced chemiluminescence reagents (Thermo Scientific, Rockford, IL), and visualized using the ChemiDoc-It imaging system (UVP). Band intensities were quantified using ImageJ.
Viability assays
CLL cells were treated with PF-04691502 for 24 hours, and viability was assessed by annexin V–fluorescein isothiocyanate (FITC) and propidium iodide (PI) negativity as previously described.39 IC50 values were measured using CalcuSyn software (Biosoft, Cambridge, United Kingdom). Peripheral blood mononuclear cells from normal donors were plated at 1 × 106 cells per milliliter, treated with PF-04691502 for 24 hours, and normal T and B cells labeled with allophycocyanin-conjugated anti-CD3 and anti-CD19, respectively, in combination with annexin V–FITC/PI.37
Eμ-TCL1 experiments
In vitro analysis using Eμ-TCL1 cells was performed at 2.5 × 106 cells per milliliter. For in vivo experiments, 1 × 107 Eμ-TCL1 leukemia cells were given by intraperitoneal injection to congenic, age-matched female C57BL/6 mice, n = 7 per group. Leukemic burden was assessed by tail bleeding, and monitoring total lymphocyte counts and the percentage of CD5+B220lo cells was done by flow cytometry. PF-04691502 was administered in 0.5% methylcellulose by oral gavage at 5 or 10 mg/kg/d. Animals were euthanized, and organs were harvested for further analysis. Total organ tumor burden was calculated by CD5+B220lo flow cytometry using Quantibrite counting beads (Life Technologies).
Results
mTOR inhibition of CLL cells augments AKTS473 signaling
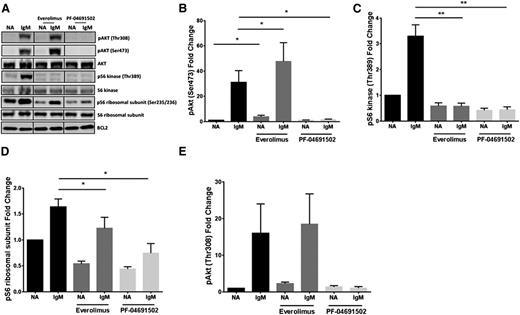
Previous studies in other tumor systems have shown that inhibition of mTOR with everolimus/rapamycin results in augmentation of the mTORC2 target, AKTS473.27,28 Here, we show that this augmentation of AKTS473 also occurs in CLL cells, both in resting conditions and more effectively after BCR engagement with soluble anti-IgM antibodies (Figure 1A-B), with the expected effects on the mTOR target, phosphorylated S6 kinase (pS6K) (Figure 1C). In contrast, the dual PI3K/mTOR inhibitor PF-04691502 does not induce hyperphosphorylation of AKTS473 and inhibits the pathway, as shown with other PI3K inhibitors (supplemental Figure 1C). pS6K was completely abrogated with PF-04691502, whereas phosphorylated S6 ribosomal protein (pS6S235/236) is regulated by both the mitogen-activated protein kinase and PI3K/mTOR signaling pathways and was therefore only partially affected by both everolimus and PF-04691502 (Figure 1D). AKT is also phosphorylated at AKTT308 in a PI3K-dependent manner by 3′-phosphoinositide-dependent kinase-1 (PDK-1). and was not significantly augmented by everolimus treatment before or after anti-IgM treatment. However, PF-04691502 completely abrogated AKTT308 phosphorylation (Figure 1E). Together, these data indicate that the same negative feedback loop involving mTORC2 is in operation in CLL cells but is circumvented by PF-04691502.
Inhibition of mTOR augments AKT signaling in CLL, which can be overcome by a dual PI3K/mTOR inhibitor. (A) CLL cells were pretreated with everolimus (1 μM) or PF-04691502 (1 μM) for 2.5 hours prior to treatment with soluble anti-IgM (IgM) for 15 minutes. Immunoblotting was performed for phosphorylated AKT (pAKTS473), S6 kinase (pS6KT389), and S6 (pS6 ribsosomal subunitS235/236). Bcl-2 was used as a loading control. The fold change in (B) pAKTS473 (n = 7), (C) pS6KT389 (n = 6), (D) pS6S235/236 (n = 7), and (E) AKTT308 (n = 5) compared to the untreated basal control after the various treatments described in panel A. *P < .05, **P < .01. Error bars represent SEM. NA, untreated basal control; SEM, standard error of the mean.
Inhibition of mTOR augments AKT signaling in CLL, which can be overcome by a dual PI3K/mTOR inhibitor. (A) CLL cells were pretreated with everolimus (1 μM) or PF-04691502 (1 μM) for 2.5 hours prior to treatment with soluble anti-IgM (IgM) for 15 minutes. Immunoblotting was performed for phosphorylated AKT (pAKTS473), S6 kinase (pS6KT389), and S6 (pS6 ribsosomal subunitS235/236). Bcl-2 was used as a loading control. The fold change in (B) pAKTS473 (n = 7), (C) pS6KT389 (n = 6), (D) pS6S235/236 (n = 7), and (E) AKTT308 (n = 5) compared to the untreated basal control after the various treatments described in panel A. *P < .05, **P < .01. Error bars represent SEM. NA, untreated basal control; SEM, standard error of the mean.
PF-04691502 reduces viability in CLL cells independently of prognostic markers
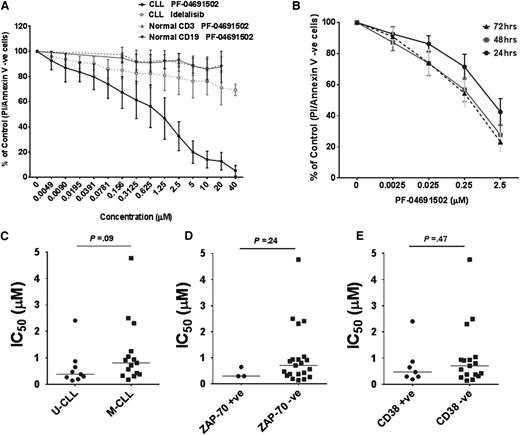
CLL samples were treated with PF-04691502, and viability was assessed using annexin V/PI staining (supplemental Figure 2A). PF-04691502 reduced viability of CLL cells after 24, 48, and 72 hours (Figure 2A-B), with a mean IC50 value of 0.96 μM (median 0.65 μM), 0.32 μM, and 0.25 μM, respectively. Each of the same CLL samples treated with idelalisib did not reach its IC50 value up to 40 μM (Figure 2A), in agreement with the literature.19,20 Normal B and T cells from age-matched controls were largely unaffected by PF-04691502 over a similar dose range. No significant differences in mean IC50 values for PF-04691502 were observed between unmutated and mutated IGHV genes (Figure 2C) or between high and low ZAP-70 (>30%) (Figure 2D) or CD38 expression (>30%) (Figure 2E). However, a trend for lower IC50 values was observed in unmutated compared to mutated IGHV genes. Furthermore, 3 CLL samples with 17p del (2 of 3 samples >70% 17p del by fluorescence in situ hybridization) were as susceptible to PF-04691502-induced death as wild-type samples (supplemental Figure 2B), indicating that PF-04691502 may also have activity independent of 17p del status.
PF-04691502 reduces cell viability of CLL cells independently of prognostic markers. (A) CLL samples (n = 25) and normal B (CD19+) and T cells (CD3+) (n = 5) were treated in the presence or absence of PF-04691502 (0.0049-40 μM) for 24 hours. Cell viability was calculated using annexin V/PI assays and measured by flow cytometry. For comparison, a proportion of the same CLL samples were treated with idelalisib (0.0049-40 μM) (n = 12). (B) CLL samples (n = 6, 3 low IC50 and 3 high IC50 at 24 hours) were treated with PF-04691502 (0.0025-2.5 μM) for 24 to 72 hours. Correlation of the response to PF-04691502 with prognostic markers (C) IGHV (n = 21), (D) ZAP-70 (n = 23), and (E) CD38 (n = 23). Error bars represent SEM. M-CLL, mutated IGHV genes; U-CLL, unmutated IGHV genes; +ve, positive; -ve, negative.
PF-04691502 reduces cell viability of CLL cells independently of prognostic markers. (A) CLL samples (n = 25) and normal B (CD19+) and T cells (CD3+) (n = 5) were treated in the presence or absence of PF-04691502 (0.0049-40 μM) for 24 hours. Cell viability was calculated using annexin V/PI assays and measured by flow cytometry. For comparison, a proportion of the same CLL samples were treated with idelalisib (0.0049-40 μM) (n = 12). (B) CLL samples (n = 6, 3 low IC50 and 3 high IC50 at 24 hours) were treated with PF-04691502 (0.0025-2.5 μM) for 24 to 72 hours. Correlation of the response to PF-04691502 with prognostic markers (C) IGHV (n = 21), (D) ZAP-70 (n = 23), and (E) CD38 (n = 23). Error bars represent SEM. M-CLL, mutated IGHV genes; U-CLL, unmutated IGHV genes; +ve, positive; -ve, negative.
Combination of isoform-specific PI3K inhibitors and an mTOR inhibitor mimic PF-04691502
PF-04691502 reduced viability of CLL cells to a greater extent compared to idelalisib. Therefore, we sought to determine whether we could replicate its efficacy using a combination of isoform-specific PI3K inhibitors, including BYL719 (PI3Kα inhibitor), GSK2636771 (PI3Kβ inhibitor), idelalisib (PI3Kδ inhibitor), and AS-605240 (PI3Kγ inhibitor), in combination with the mTOR inhibitor everolimus (mTORC1 inhibitor). Each inhibitor alone (1 μM) reduced viability by no more than ∼10% (supplemental Figure 2C). This result was in contrast to PF-04691502, which at the same concentration reduced CLL cell viability by >50%. The combination of all 5 selective inhibitors at 1 μM significantly reduced CLL cell viability more than any of the inhibitors alone, albeit significantly less than PF-04691502 (P = .004; n = 11). Lastly, we showed that a combination of idelalisib with everolimus was significantly more efficacious than either agent alone (P = .01 and P = .02, respectively) (supplemental Figure 2D) but significantly less so than PF-04691502 (P = .03). Together, these data suggest that PF-04691502 likely achieves its robust killing effects through simultaneous inhibition of mTORC1, mTORC2, and PI3Kδ.
PF-04691502 induces apoptosis in a caspase-dependent mechanism
To determine the mechanism of death induced by PF-04691502, we first investigated expression of the key proapoptotic BH3-only proteins Noxa, Puma, Bad, Bim, and Bax, and the antiapoptotic Bcl-2 family proteins Mcl-1 and Bcl-2, which regulate apoptosis within CLL cells. Incubation with PF-04691502 (0.31-1.25 μM) induced Noxa and Puma expression (protein and messenger RNA) in a dose-dependent manner (Figure 3A; data not shown). No significant changes were observed for Bim, Bad, Bcl-2, or Mcl-1 (data not shown). Basal pAKTS473 and pS6KT389 were inhibited by PF-04691502, as expected. In response to proapoptotic stimuli, Bax undergoes a conformational change and subsequently becomes inserted into the outer mitochondrial membrane where it facilitates cytochrome c release and subsequent apoptosis. Using the conformational change–specific 6A7 antibody,37 we showed that CLL cells incubated with PF-04691502 produced a marked increase in the active, mitochondrially resident conformation of Bax (Figure 3B). Furthermore, treatment of CLL cells with PF-04691502 (0.31-5 μM) for 18 hours induced caspase-3 cleavage and subsequent appearance of the p85 cleaved subfragment of poly (ADP-ribose) polymerase (PARP) (Figure 3C). To confirm PF-04691502-induced apoptosis of CLL cells in a caspase-dependent manner, we treated cells with the pan-caspase inhibitor ZVAD.fmk. ZVAD.fmk significantly prevented the PF-04691502-induced decrease in cell viability, even at the highest concentrations of PF-04691502 (20 μM; P < .001; n = 7) (Figure 3D).
PF-04691502 induces the intrinsic apoptosis pathway after PI3K/mTOR pathway inhibition. (A) Immunoblotting was used to show expression of the Bcl-2 family of proapoptotic proteins, namely Noxa and Puma, in resting nonactivated cells. pAKTS473 and pS6KT389 were used to confirm that PF-04691502 treatment (18 hours) had inhibited the PI3K/mTOR pathway. HSC70 was used as a loading control. The figure is representative of 9 independent experiments. (B) BAX conformational change was investigated in the presence or absence of PF-04691502; immunoprecipitation (IP) with the BAX 6A7 antibody was performed as described in supplemental Methods). IgH and IgL were included to confirm that identical quantities of antibody were used for the immunoprecipitation. Blot is representative of 4 independent experiments. (C) Cleavage of caspase 3 and its substrate PARP (marker of apoptosis). Blot is representative of 10 independent experiments. (D) CLL samples (n = 7) were treated with PF-04691502 as above but with or without the pan-caspase inhibitor ZVAD.fmk and analyzed by flow cytometry for annexin V/PI negativity. (E) Stromal fibroblasts (HFFF2) were cocultured with CLL cells and the whole well was treated in the presence or absence of continuous PF-04691502 (2.5 μM) for 24 hours. CLL cells were removed by scraping (or by pipetting; see supplemental Figure 3D), and cell viability was analyzed using annexin V/PI analysis by flow cytometry (n = 7). (F) The assay was performed as in panel E, except that the CLL cells were pretreated for 1 hour with PF-04691502 prior to washing out the drug, and the treated CLL cells were plated into wells containing stromal fibroblasts (n = 7) (described in supplemental Methods). Error bars represent SEM. No difference was observed between scraping all cells (panel E) or washing off only the CLL cells (see supplemental Figure 3D). *P < .05, **P < .01. NS, not significant.
PF-04691502 induces the intrinsic apoptosis pathway after PI3K/mTOR pathway inhibition. (A) Immunoblotting was used to show expression of the Bcl-2 family of proapoptotic proteins, namely Noxa and Puma, in resting nonactivated cells. pAKTS473 and pS6KT389 were used to confirm that PF-04691502 treatment (18 hours) had inhibited the PI3K/mTOR pathway. HSC70 was used as a loading control. The figure is representative of 9 independent experiments. (B) BAX conformational change was investigated in the presence or absence of PF-04691502; immunoprecipitation (IP) with the BAX 6A7 antibody was performed as described in supplemental Methods). IgH and IgL were included to confirm that identical quantities of antibody were used for the immunoprecipitation. Blot is representative of 4 independent experiments. (C) Cleavage of caspase 3 and its substrate PARP (marker of apoptosis). Blot is representative of 10 independent experiments. (D) CLL samples (n = 7) were treated with PF-04691502 as above but with or without the pan-caspase inhibitor ZVAD.fmk and analyzed by flow cytometry for annexin V/PI negativity. (E) Stromal fibroblasts (HFFF2) were cocultured with CLL cells and the whole well was treated in the presence or absence of continuous PF-04691502 (2.5 μM) for 24 hours. CLL cells were removed by scraping (or by pipetting; see supplemental Figure 3D), and cell viability was analyzed using annexin V/PI analysis by flow cytometry (n = 7). (F) The assay was performed as in panel E, except that the CLL cells were pretreated for 1 hour with PF-04691502 prior to washing out the drug, and the treated CLL cells were plated into wells containing stromal fibroblasts (n = 7) (described in supplemental Methods). Error bars represent SEM. No difference was observed between scraping all cells (panel E) or washing off only the CLL cells (see supplemental Figure 3D). *P < .05, **P < .01. NS, not significant.
PF-04691502 reduced viability of CLL cells in coculture with stromal cells
CLL cells are known to receive key survival signals from stromal cells in the bone marrow and lymph nodes, reducing CLL apoptosis and promoting drug resistance.12 Therefore, we investigated the effect of PF-04691502 on CLL cells cocultured with human stromal fibroblast cells (HFFF2), which we have observed can provide antiapoptotic signaling to CLL cells (Dias et al, manuscript in preparation). PF-04691502 significantly inhibited signaling induced by HFFF2 (supplemental Figure 3A) and reduced CLL cell viability (P = .016), thus showing its ability to overcome stromal support (Figure 3E). To preclude that its activity was due to a direct cytotoxic effect on the fibroblasts (supplemental Figure 3B), we also performed “wash-out” experiments whereby PF-04691502 was added to the CLL cells for 1 hour and washed off prior to addition to the HFFF2 cells. Cell viability was still significantly reduced by PF-04691502 (P = .016), and downstream signaling was inhibited in this setting (Figure 3F; supplemental Figure 3C).
Dual PI3K/mTOR inhibition decreases prosurvival BCR signaling
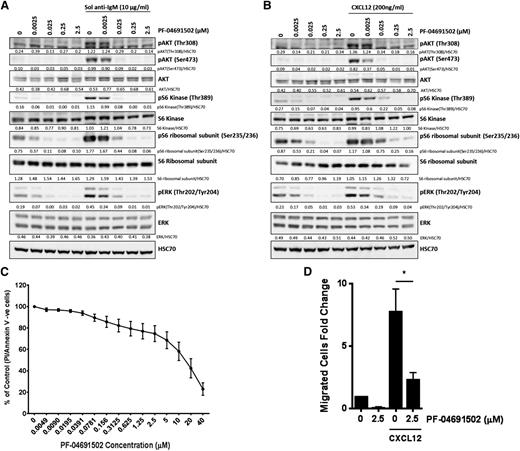
Ligation of the CLL BCR by antigen/autoantigen is thought to primarily occur in the lymph nodes, acting to enhance CLL survival and resistance to chemotherapy.40 Antigen in lymph nodes may be present in both soluble and membrane-bound forms. Therefore, we used both soluble and bead-immobilized anti-IgM41 to investigate the effect of PF-04691502 (2.5-2500 nM) on BCR signaling. Analysis of downstream signaling showed that both the soluble (Figure 4A) and bead-immobilized (Figure 4B) anti-IgM induced strong activation of the pAKTS473/T308, pERKT202/Y204, pS6KT389, and pS6S235/236 ribosomal subunit. PF-04691502 significantly inhibited pAKTS473/T308 and pS6KT389 in CLL samples after soluble and immobilized anti-IgM treatment and at concentrations below maximum achievable plasma drug concentrations (100-200 nM).34 pERKT202/Y204 and pS6S235/236 were significantly inhibited by PF-04691502 after soluble (Figure 4A) but not bead-immobilized (Figure 4B) anti-IgM treatment. These data indicate that PF-04691502 inhibits PDK-1-mediated pAKTT308, mTORC1-induced pS6KT389, and mTORC2-mediated pAKTS473 activation. Furthermore, activation of these BCR signals with bead-immobilized anti-IgM strongly protects CLL cells from apoptosis, as indicated by a decrease in cleaved caspase 3 and PARP compared to the control, but was reversed upon treatment with PF-04691502 (supplemental Figure 4A).
PF-04691502 inhibits anti-IgM–induced signaling. CLL cells were treated with (A) soluble anti-IgM beads (n = 9) and (B) immobilized (Imm) anti-IgM beads (n = 5) prior to evaluation of downstream signaling in the presence and absence of PF-04691502 (0.0025-2.5 μM) by immunoblot analysis. Representative cases are shown. CLL cells were treated for 1 hour with PF-04691502 prior to soluble/immobilized anti-IgM addition. pAKTT308 and pS6KT389 were used as markers of PI3K signaling, pAKTS473 and pS6S235/236 were used as markers of mTOR signaling, and pERKT202/Y204 was used as a marker of mitogen-activated protein kinase signaling. HSC70 was used as a loading control.
PF-04691502 inhibits anti-IgM–induced signaling. CLL cells were treated with (A) soluble anti-IgM beads (n = 9) and (B) immobilized (Imm) anti-IgM beads (n = 5) prior to evaluation of downstream signaling in the presence and absence of PF-04691502 (0.0025-2.5 μM) by immunoblot analysis. Representative cases are shown. CLL cells were treated for 1 hour with PF-04691502 prior to soluble/immobilized anti-IgM addition. pAKTT308 and pS6KT389 were used as markers of PI3K signaling, pAKTS473 and pS6S235/236 were used as markers of mTOR signaling, and pERKT202/Y204 was used as a marker of mitogen-activated protein kinase signaling. HSC70 was used as a loading control.
PF-04691502 inhibits CXCL12-induced signaling and subsequent migration
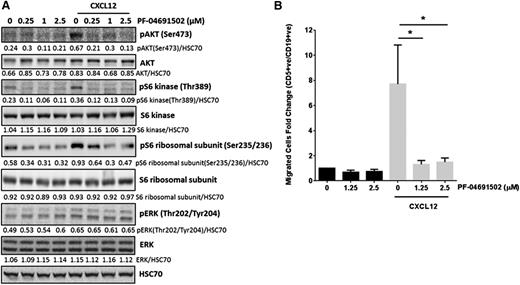
In vivo, CXCL12 signaling enables CLL cells to enter and become retained within protective lymph node microenvironments.12 Therefore, disruption of CXCL12/CXCR4 signaling provides an attractive therapeutic target.12 Here, CLL cells treated with CXCL12 (200 ng/mL) for 10 minutes showed increased pAKTS473, pS6KT389, and pS6S235/236, which was reversed after treatment with PF-04691502 (Figure 5A). In contrast, pERKT202/Y204 was not affected by PF-04691502 pretreatment, potentially due to downstream signaling from Syk/Ras being unaffected by PF-04691502. Next, we determined the effect of PF-04691502 on CLL motility in an in vitro transwell migration assay. Treatment of CLL cells with PF-04691502 showed no significant effect on basal CLL motility; however, CXCL12-induced CLL motility was significantly abrogated after pretreatment with PF-04691502 for only 30 minutes (P = .02 and P = .025 at 1.25 and 2.5 μM, respectively) (Figure 5B) and was not dependent on reduced expression of CXCR4 (supplemental Figure 4B). These data indicate that PF-04691502 inhibits CXCL12 signaling and subsequent migration, which has potential importance for CLL cells accessing the protective lymph node microenvironment in vivo.
PF-04691502 inhibits CXCL12 signaling and subsequent migration. (A) CLL cells were treated with 200 ng/mL CXCL12 for the times indicated in the presence or absence of PF-04691502 (0.25-2.5 μM). PF-04691502 was added 30 minutes prior to the addition of CXCL12. Immunoblotting was performed for pAKTS473, pS6KT389, pS6S235/236, pERKT202/Y204, total proteins, and the loading control HSC70. Blot is representative of 6 independent experiments. (B) Using a transwell migration assay, we evaluated the migration of CLL cells toward 200 ng/mL CXCL12 in the presence or absence of PF-04691502 (1.25-2.5 μM). Flow cytometry was used to count the number of CD5+CD19+cells that had passed through the transwell filter (n = 5) (see supplemental Methods). Error bars represent SEM. *P < .03.
PF-04691502 inhibits CXCL12 signaling and subsequent migration. (A) CLL cells were treated with 200 ng/mL CXCL12 for the times indicated in the presence or absence of PF-04691502 (0.25-2.5 μM). PF-04691502 was added 30 minutes prior to the addition of CXCL12. Immunoblotting was performed for pAKTS473, pS6KT389, pS6S235/236, pERKT202/Y204, total proteins, and the loading control HSC70. Blot is representative of 6 independent experiments. (B) Using a transwell migration assay, we evaluated the migration of CLL cells toward 200 ng/mL CXCL12 in the presence or absence of PF-04691502 (1.25-2.5 μM). Flow cytometry was used to count the number of CD5+CD19+cells that had passed through the transwell filter (n = 5) (see supplemental Methods). Error bars represent SEM. *P < .03.
PF-04691502 has efficacy in murine Eμ-TCL1 CLL cells ex vivo
Next, we examined whether PF-04691502 displayed activity against murine Eμ-TCL1 tumor cells isolated and cultured ex vivo. Eμ-TCL1 tumor cells were isolated from the spleens of tumor-bearing mice and then treated with anti-IgM or CXCL12 in the presence or absence of PF-04691502, and the effects on downstream signaling were investigated by immunoblot analysis. PF-04691502 substantially blocked signaling induced by both stimuli, reducing pAKTT308, pAKTS473, pS6KT389, and pS6S235/236 to below background levels, with lesser effects on pERKT202/Y204 at 2.5 to 25 nM PF-04691502 (Figure 6A-B). These effects translated into an ability of PF-04691502 to efficiently kill Eμ-TCL1 tumor cells (n = 8 different tumors) (Figure 6C) and inhibit migration toward CXCL12 (Figure 6D). These data recapitulated those seen with primary human CLL cells above.
PF-04691502 inhibits anti-IgM– and CXCL12–induced signaling in Eμ-TCL1 murine cells. Eμ-TCL1 cells were removed from mice and grown in vitro. Eμ-TCL1 cells were treated with soluble (Sol) anti-IgM (5-15 minutes) (A) or 200 ng/mL CXCL12 (B) in the presence or absence of PF-04691502 (0.0025-2.5 μM), and the effects on downstream signaling (pAKTT308, pAKTS473, pS6KT389, pS6S235/236, and pERKT202/Y204) were investigated by immunoblotting. HSC70 was used as a loading control. (C) Viability of Eμ-TCL1 cells by PF-04691502 was evaluated by annexin V/PI assays and represented as percentage viable cells. (D) Migration was performed as previously described in Figure 5B. Error bars represent SEM. *P < .02.
PF-04691502 inhibits anti-IgM– and CXCL12–induced signaling in Eμ-TCL1 murine cells. Eμ-TCL1 cells were removed from mice and grown in vitro. Eμ-TCL1 cells were treated with soluble (Sol) anti-IgM (5-15 minutes) (A) or 200 ng/mL CXCL12 (B) in the presence or absence of PF-04691502 (0.0025-2.5 μM), and the effects on downstream signaling (pAKTT308, pAKTS473, pS6KT389, pS6S235/236, and pERKT202/Y204) were investigated by immunoblotting. HSC70 was used as a loading control. (C) Viability of Eμ-TCL1 cells by PF-04691502 was evaluated by annexin V/PI assays and represented as percentage viable cells. (D) Migration was performed as previously described in Figure 5B. Error bars represent SEM. *P < .02.
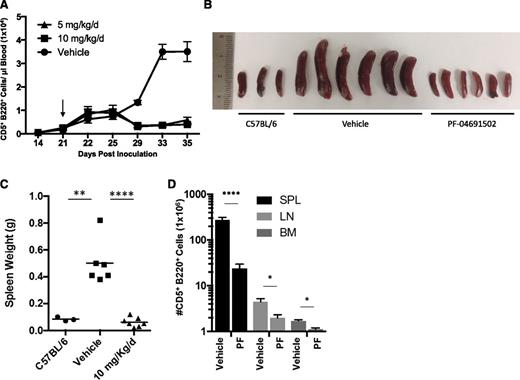
In vivo efficacy of PF-04691502
Lastly, we explored the activity of PF-04691502 in vivo. Cohorts of mice were inoculated with Eμ-TCL1 tumor cells and, when tumor was visible in the blood (∼21 days), mice were randomized to receive 5 or 10 mg/kg/d PF-04691502 or vehicle control by oral gavage. The leukemic burden in the blood was monitored over the following 14 days (Figure 7A). After receiving the drug, a small but significant lymphocytosis was observed, which persisted for ∼4 to 7 days. Subsequently, PF-04691502 elicited a marked reduction in leukemic burden compared to the vehicle control (P = .0001), indicating a profound antitumor effect. Both drug doses demonstrated the same efficacy, indicating that the minimum effective dose was achieved. Fourteen days posttreatment, vehicle-recipient animals exhibited a terminal disease and were euthanized alongside mice receiving 10 mg/kg/d PF-04691502, and the effects of the drug on multiple tissues, including spleen, lymph nodes, and bone marrow (Figure 7B-D), were determined. Mice receiving 5 mg/kg/d were maintained on drug for survival experiments. Visual assessment and weighing of the spleen illustrated the ability of the drug to prevent tumor expansion at this site (Figure 7B-C), which was confirmed by enumerating the number of tumor cells (Figure 7D). No increase in splenic mass was observed as compared to normal healthy C57BL/6 mice, whereas vehicle-treated mice exhibited a fourfold to eightfold increase in splenic mass, in addition to a tumor cell count an order of magnitude higher than that in PF-04691502-treated mice. Next, we examined the macroscopic appearance of the spleen using hematoxylin-and-eosin staining (supplemental Figure 5A). These images indicate that the Eμ-TCL1 leukemias destroy the typical splenic architecture, whereas this was largely prevented by PF-04691502 treatment. In addition to the spleen, we also observed similar effects of the drug reducing tumor expansion in the lymph nodes and bone marrow (Figure 7D). Cumulatively, these effects significantly enhanced survival in 5 mg/kg/d–recipient animals (P ≤ .0001) (supplemental Figure 5B). Furthermore, mice showed a small but significant reduction in normal B-cell number but no significant reduction in normal T-cell number (data not shown). These data indicate that PF-04691502 has substantial efficacy in vivo, warranting further investigation of dual PI3K/mTOR inhibitors for the treatment of CLL.
The effect of PF-04691502 in the Eμ-TCL1 mouse model. Mice inoculated with Eμ-TCL1 tumor cells were treated by oral gavage with 5 or 10 mg/kg/d PF-04691502 or placebo control over 14 days of continual treatment after the emergence of a leukemic phase occurred at day 21 (depicted by the arrow in panel A). (A) Peripheral leukemic (CD5+CD220+) cell number was evaluated by flow cytometry in the presence of PF-04691502 or the vehicle control. (B) Illustration of the spleen sizes from mice treated with 10 mg/kg/d PF-04691502 or vehicle control as compared to the wild-type mouse C57BL/6. (C) Weights of the spleens depicted in panel B. (D) Leukemic cell number was also investigated in various organs, including spleen (SPL), lymph nodes (LN), and bone marrow (BM) after treatment with PF-04691502 or the vehicle control. Error bars represent SEM. *P < .02, **P < .004, ****P < .0001. PF, PF-04691502.
The effect of PF-04691502 in the Eμ-TCL1 mouse model. Mice inoculated with Eμ-TCL1 tumor cells were treated by oral gavage with 5 or 10 mg/kg/d PF-04691502 or placebo control over 14 days of continual treatment after the emergence of a leukemic phase occurred at day 21 (depicted by the arrow in panel A). (A) Peripheral leukemic (CD5+CD220+) cell number was evaluated by flow cytometry in the presence of PF-04691502 or the vehicle control. (B) Illustration of the spleen sizes from mice treated with 10 mg/kg/d PF-04691502 or vehicle control as compared to the wild-type mouse C57BL/6. (C) Weights of the spleens depicted in panel B. (D) Leukemic cell number was also investigated in various organs, including spleen (SPL), lymph nodes (LN), and bone marrow (BM) after treatment with PF-04691502 or the vehicle control. Error bars represent SEM. *P < .02, **P < .004, ****P < .0001. PF, PF-04691502.
Discussion
Treatment of CLL patients with ibrutinib and idelalisib have produced impressive clinical responses; however, the agents are not curative and only suppress the disease. Therefore, identifying an agent that can purge CLL cells from the protective lymph node and bone marrow niches in combination with substantive tumor toxicity may prove essential for an eventual cure. Our results showed that PF-04691502 inhibited BCR- and CXCL12-induced signaling, resulting in caspase-dependent apoptosis of CLL cells in vitro at nanomolar concentrations, whereas treatment of normal B and T cells resulted in little to no death. Caspase-dependency was confirmed with the pan-caspase inhibitor ZVAD.fmk and was preceded by the proapoptotic conformational change in Bax. Bax activation correlated with the induction of the BH3-only Bcl-2 family members Noxa and Puma, suggesting activation of the intrinsic apoptotic pathway. However, because 17p del cases displayed equivalent sensitivity to PF-04691502 as wild-type samples, the pathway leading to Noxa and Puma induction after PF-04691502 treatment may be TP53 independent. The mechanism behind the Noxa and Puma induction is currently unknown and the subject of our ongoing studies. PF-04691502 induced apoptosis independently of known prognostic markers, which is consistent with findings with idelalisib in CLL.19 However, an intriguing finding was the enhanced apoptotic potency of PF-04691502 in comparison to idelalisib.19,42 This increased cytotoxicity against CLL cells by PF-04691502 may be due to inhibition of >1 PI3K isoform or more likely due to the co-inhibition of PI3K and mTORC1/2. Evidence in mantle cell lymphoma indicates that constitutive PI3Kα signaling limits the efficacy of PI3Kδ selective inhibitors.43 Functional redundancy between PI3K isoforms in the survival of neutrophils has previously been shown15 ; therefore, it may be theorized that in CLL cells, other PI3K isoforms may compensate for the selective inhibition of PI3Kδ with idelalisib, resulting in lower levels of cytotoxicity with this drug. However, the γ/δ inhibitor IPI-145 did not increase CLL cell apoptosis more than idelalisib in vitro.44 In contrast, the dual PI3Kα/γ inhibitor PIK90 and the pan-PI3K inhibitor NVP-BKM120 (buparlisib) induce substantially more CLL cell apoptosis23,45 than single-isoform inhibitors.19 The potential caveat to inhibition of more than a single PI3K isoform is the possibility of greater toxicity to normal tissues. However, a number of pan-PI3K inhibitors and dual PI3K/mTOR inhibitors are currently progressing through clinical trials for various malignancies with promising results.46 Interestingly, long-term PI3Kα inhibition was not detrimental to mice and, in fact, protected against a reduction in insulin sensitivity, glucose tolerance, and fat accumulation.47 During our study, we combined inhibitors that targeted specific isoforms of PI3K and mTOR, replicating our findings with PF-04691502. Although combining the PI3Kα/β/δ/γ inhibitors or idelalisib with the mTOR inhibitor everolimus induced substantial apoptosis, PF-04691502 alone still induced significantly more. This may be because everolimus inhibits predominately mTORC1, whereas PF-04691502 inhibits both mTORC1 and mTORC2, which is essential to inhibit the positive feedback and subsequent phosphorylation of AKTS473. However, these data suggest that an mTORC1 inhibitor, and maybe more importantly, a dual mTORC1/2 inhibitor, in combination with idelalisib may be more therapeutically beneficial in the treatment of CLL than PI3K inhibition alone.
Ligation of the BCR occurs primarily in the protective lymph node microenvironment48 and is thought to be crucial for survival of the malignant clone.40 Furthermore, CXCL12 induces migration of the CLL cells from the periphery into the lymph node, promoting their retention within protective microenvironments.12 CXCL12/CXCR4 signaling has previously been shown to inhibit spontaneous cell death and chemotherapy-induced apoptosis.49 Therefore, our finding that PF-04691502 could overcome prosurvival anti-IgM–induced signaling and substantially inhibit CXCL12 signaling and subsequent CLL migration is of clear importance. This finding is also in agreement with what has been described for idelalisib.20 These results indicate that PF-04691502 may have similar clinical characteristics to other kinase inhibitors, where the inhibition of BTK, PI3Kδ, or SYK results in reduced interaction of CLL cells with their protective microenvironments, redistribution of the tumor cells from tissues to the blood, and some cellular toxicity.19,50 These previous effects resulted in clinical activity whereby patients underwent rapid lymph node shrinkage and lymphocytosis within weeks of treatment initiation.7,11,20 Due to the relatively low toxicity of these agents alone on CLL cells, combining them with Bcl-2 antagonists (ABT-199) has been proposed. However, given the trend for greater Mcl-1 expression in CLL patient lymph nodes,51 which is regulated by microenvironmental factors through PI3K, PI3K inhibition may have greater efficacy. These latter results raise the question as to whether targets downstream of PI3K, such as AKT, are equally tractable. However, current data suggest not, because CLL cells treated with AKT inhibitors (MK2206 and AZD5363) required concentrations in excess of 10 μM to elicit relatively small amounts of apoptosis, and clinical trials have stopped recruiting (NCT01369849).
Tumor cell redistribution was also evident in the Eμ-TCL1 model, with a transient lymphocytosis seen in the blood concomitant with reduced tumor load in the secondary lymphoid organs and bone marrow upon PF-04691502 treatment. Such lymphocytosis is likely a consequence of reduced chemokine receptor expression or signal inhibition. Interestingly, although PF-04691502 treatment prevented accumulation of tumor in the spleen and caused protection of the global splenic architecture, tumor was still evident, even in the presence of PF-04691502. Therefore, these tumor deposits likely reflect treatment-resistant reservoirs that require additional interventions with other treatment modalities, which is the basis for our ongoing studies.
The results from this study show that simultaneous inhibition of all 4 class-1 PI3K isoforms in combination with the inhibition of mTOR kills CLL cells more potently than selective PI3Kδ inhibition alone. The data generated with systems mimicking ongoing BCR stimulation and stromal support indicate the ability of PF-04691502 to overcome protective microenvironment signaling in vivo. These results are supported by in vivo experimentation using the Eμ-TCL1 mouse model that further indicates that PF-04691502 may prove therapeutically useful for the treatment of CLL. Further studies are now required to investigate the key modes of action of PF-04691502 and explore its utility in combination with other treatment modalities.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
The authors thank Pfizer for providing pure substance (PF-04691502) for this study; the patients for supplying tissue; Dr Duriez for providing annexin V–FITC; Dr Egle, Dr Pekarsky, and Professor Croce for providing the Eμ-TCL1 mouse model; Professor Thomas for providing the HFFF2 cells; Mr Tracy and Mrs Henderson for biobanking and characterization of the CLL samples used in this study; and Dr Gobessi and Professor Efremov for advice on the use of the Eµ-TCL1 model.
This work was supported by grants from Leukaemia and Lymphoma Research (12021, 12044), Kay Kendall (KKL687), the Experimental Cancer Medicine Centre (C24563/A15581), and the Cancer Research United Kingdom Centre (C34999/A18087).
Authorship
Contribution: M.D.B., M.J.C., J.C.S., M.S.C., and A.J.S. designed the research and wrote the manuscript; M.D.B., M.J.C., M.L., L.D.S., M.A.-H., K.L.C., T.T., M.R., E.L., S.D., and S.M. performed the research and analyzed the data; F.F. obtained consent from patients, obtained blood samples, and collated and analyzed the clinical data; A.D. provided the patients and clinical data; F.K.S., P.W.M.J., and G.P. provided critical review, suggested experiments, and edited the paper.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Andrew J. Steele, Cancer Sciences Unit, Somers Building (Mailpoint 824), Southampton General Hospital, Tremona Rd, Southampton SO16 6YD, United Kingdom; e-mail: a.steele@soton.ac.uk.
References
Author notes
M.D.B. and M.J.C. contributed equally to this study.



This feature is available to Subscribers Only
Sign In or Create an Account Close Modal