Key Points
Modulation of thrombin-dependent platelet activation by TFPI is required for successful embryonic development.
TFPI dampens intravascular thrombin generation even in the absence of thrombin-mediated platelet activation.
Abstract
Tissue factor pathway inhibitor (TFPI) is a critical anticoagulant protein present in endothelium and platelets. Mice lacking TFPI (Tfpi−/−) die in utero from disseminated intravascular coagulation. They are rescued by concomitant tissue factor (TF) deficiency, demonstrating that TFPI modulates TF function in vivo. Recent studies have found TFPI inhibits prothrombinase activity during the initiation of coagulation and limits platelet accumulation during thrombus formation, implicating TFPI in modulating platelet procoagulant activity. To examine whether altered platelet function would compensate for the lack of TFPI and rescue TFPI-null embryonic lethality, Tfpi+/− mice lacking the platelet thrombin receptor, protease activated receptor 4 (PAR4; Par4−/−), or its coreceptor, PAR3, were mated. PAR3 deficiency did not rescue Tfpi−/− embryos, but >40% of expected Tfpi−/−:Par4−/− offspring survived to adulthood. Adult Tfpi−/−:Par4−/− mice did not exhibit overt thrombosis. However, they had focal sterile inflammation with fibrin(ogen) deposition in the liver and elevated plasma thrombin-antithrombin complexes, indicating activation of coagulation at baseline. Tfpi−/−:Par4−/− mice have platelet and fibrin accumulation similar to Par4−/− mice following venous electrolytic injury but were more susceptible than Par4−/− mice to TF-induced pulmonary embolism. In addition, ∼30% of the Tfpi−/−:Par4−/− mice were born with short tails. Tfpi−/−:Par4−/− mice are the first adult mice described that lack TFPI with unaltered TF. They demonstrate that TFPI physiologically modulates thrombin-dependent platelet activation in a manner that is required for successful embryonic development and identify a role for TFPI in dampening intravascular procoagulant stimuli that lead to thrombin generation, even in the absence of thrombin-mediated platelet activation.
Introduction
Tissue factor pathway inhibitor (TFPI) is a multivalent Kunitz-type protease inhibitor that exerts anticoagulant activity through inhibition of the blood coagulation proteases factor VIIa (fVIIa) and factor Xa (fXa).1 By inhibiting these proteases, TFPI blocks the activity of 2 potent procoagulant enzyme complexes: (1) the tissue factor (TF)-fVIIa complex1,2 and (2) early forms of the prothrombinase complex consisting of fXa and partially B-domain cleaved forms of factor Va (fVa).3 A human completely lacking TFPI has not been described, and TFPI knockout mice homozygous for the null allele (Tfpitm1Gjb; Tfpi−/−) die in utero,4 highlighting the importance of TFPI anticoagulant activity during development. The embryonic lethal phenotype is rescued by concomitant deficiency of fVII5 or markedly decreased expression of TF,6 demonstrating that TFPI directly counterbalances TF-fVIIa activity during embryonic development. The ability of TFPIα to inhibit platelet prothrombinase activity during the initiation of coagulation is a more recently recognized anticoagulant function of TFPI.3 It remains unknown whether concomitant deficiency of platelet activation will rescue Tfpi−/− mice from embryonic lethality.
Thrombin promotes blood coagulation through generation of fibrin and activation of platelets. It activates mouse platelets by cleaving protease activated receptors (PARs)3 and 4 on the mouse platelet surface.7 PAR3 contains a hirudin-like binding region that binds thrombin, but this does not result in downstream signaling through PAR3. Conversely, PAR4 can signal but does not contain a hirudin-like binding region. Instead, PAR3 acts a coreceptor for PAR4 activation by localizing thrombin to the platelet membrane in the vicinity of PAR4.8 Therefore, platelet activation through PAR4 in the absence of PAR3 requires higher thrombin concentration than when both receptors are present.8 Thrombin-mediated activation of murine platelets does not occur in the absence of PAR4.7
TFPI is produced predominantly by the endothelium.9 However, it is also produced by megakaryocytes and is present within platelets.10,11 The importance of hematopoietic cell TFPI is demonstrated in a mouse model of hemophilia, where its absence improves the bleeding phenotype of these mice.12 In addition, hematopoietic cell TFPI dampens thrombus volume after electrolytic vascular injury by limiting platelet accumulation without effect on fibrin accumulation.13 This effect on platelet accumulation, but not fibrin accumulation, is similar to that observed when Par4−/− mice are subjected to vascular injury.14
As platelet TFPI inhibits prothrombinase3 and limits platelet accumulation at sites of vascular injury,13 we hypothesized that a lack of platelet responsiveness to thrombin would compensate for the lack of TFPI and rescue the embryonic lethal phenotype associated with TFPI deficiency. To test this hypothesis, Par3−/− and Par4−/− mice were bred into Tfpi+/− mice. Their F1 offspring were subsequently mated and examined for surviving Tfpi−/− pups. Deficiency of PAR3 did not rescue the embryonic lethality of Tfpi−/− embryos. In contrast, PAR4 deficiency rescued nearly half of Tfpi−/− embryos to adulthood.
Methods
Generation of mice
Mice heterozygous for the TFPI-null allele (Tfpitm1Gjb;Tfpi+/−) were from Dr George Broze, Jr (Washington University, St Louis, MO). PAR3- and PAR4-deficient mice (F2rl2−/−;Par3−/− and F2rl3−/−;Par4−/−) were from Dr Shaun Coughlin (University of California, San Francisco, CA). All strains of mice were backcrossed on the C57Bl/6 background for ≥10 generations. Tfpi+/− mice were mated with Par3−/− or Par4−/− mice to produce doubly heterozygous mice. Mice of the same PAR genotype were then mated to produce Tfpi+/−:Par3−/− and Tfpi+/−:Par4−/− mice. Tfpi+/− mice of the same PAR genotype were then mated, and the offspring were genotyped to determine whether the lack of either PAR3 or PAR4 rescued the embryonic lethal phenotype of the TFPI-null embryos. All experimental mice were male and 8 to 12 weeks old unless otherwise indicated.
Blood collection and sample preparation
Mice were anesthetized using ketamine/xylazine, and blood was collected via the inferior vena cava (IVC) into 3.2% citrate (9:1 ratio) using a 27-G needle and syringe. For calibrated automated thrombography (CAT) assays, blood was drawn into 3.2% citrate (9:1 ratio) and corn trypsin inhibitor (50 µg/mL). Platelet poor plasma (PPP) was prepared by centrifugation of blood at 7500g for 10 minutes at room temperature, and the collected plasma was then centrifuged at 20 000g for 10 minutes at 4°C.
Complete blood counts
Complete blood counts (CBCs) were determined using an animal blood counter (scil Vet abc Plus).
Mouse tail length
At 8 weeks of age, the length of the tail, from the tail tip to the point at which the tail meets the body, was measured in millimeters. The mouse body length, from the nose to the point at which the tail meets the body, was also measured, and the ratio of the body length to tail length was determined. The tail length was measured in mice with straight tails only.
Quantitation of thrombin-antithrombin complexes and d-dimers
Thrombin-antithrombin (TAT) complexes (Enzygnost TAT Micro kit; Siemens Healthcare, Malvern, PA) and d-dimers (Asserachrom d-di kit; Diagnostica Stago, Parsippany, NJ) were quantified in PPP, according to the manufacturers’ instructions.
CAT
Thrombin generation in PPP from age-matched wild-type, Par4−/−, and Tfpi−/−:Par4−/− mice was assessed by CAT. Briefly, PPP from 2 mice of the same genotype was pooled and then diluted 1:4 with 4-(2-hydroxyethyl)-1-piperazineethanesulfonic acid (HEPES) buffered saline (50 mM HEPES and 100 mM sodium chloride, pH 7.4). Forty microliters of this sample was added to 10 µL of 0.1 pM tissue factor (TF; Hemoliance Recombiplastin, Instrumentation Laboratory) and 4 µM phospholipid (phosphotidylcholine, phosphotidylserine, and phosphotidylethanolamine at a 60:20:20 ratio; Avanti Polar Lipids, Alabaster, AL) and incubated for 10 minutes at 37°C. The reaction was initiated with 10 µL of FluCa substrate (Diagnostica Stago), and thrombin generation was monitored over 2 hours using a Fluoroskan Ascent plate reader (ThermoScientific, Waltham, MA) with 10-second intervals between readings. Data analysis was performed using Thrombinoscope software (Thrombinoscope, Maastricht, The Netherlands).
Histology
Mice were anesthetized with ketamine/xylazine, and the major organs were removed and then fixed in 10% formalin. Tissues were paraffin embedded and cut into 5-µm sections. The following antibodies were used for immunohistochemistry: anti-CD3 (AB828; Abcam, Cambridge, MA); anti-Fibrin(ogen) (A0080; Dako, Carpinteria, CA); anti-F4/80, and anti-Ly6B.2 alloantigen (MCA497G and MCA771G, both from AbD Serotec, Raleigh, NC). Biotinylated, species-specific secondary antibodies, and either streptavidin-horseradish peroxidase or streptavidin-alkaline phosphatase and enzyme-specific substrates were used for detection. Slides stained with secondary antibody only served as a negative control. Liver lesion burden of Tfpi−/−:Par4−/− and Par4−/− mice (n = 3, age < 3 months for each genotype) was quantified using a calibrated reticle. More than 15 fields per liver section were counted and averaged for each mouse to obtain the number of lesions per square millimeter.
Electrolytic venous injury model
Electrolytic injury of the femoral vein was performed as previously described.15 Briefly, the femoral vein was exposed in pentobarbital-anesthetized mice (50 mg/kg), and its surface was injured by a 30-second electrolytic injury to a localized spot. Five minutes before the injury, rhodamine 6G (0.5 mg/kg, to label platelets in vivo) and AlexaFluor647-labeled anti-fibrin antibody were injected into mice via the external jugular vein in a 100-μL volume. The anti-fibrin antibody was purified from ascites obtained from a hybridoma clone (59D8) kindly provided by Dr Marschall Runge. Localization of each clotting element at the clot site was quantified over time through time-lapse image capture and subsequent offline analysis.
TF-induced pulmonary embolism model
Mice were weighed and anesthetized with ketamine/xylazine, and the IVC was exposed. A 1/40 dilution of TF (diluted in phosphate-buffered saline + 0.1% bovine serum albumin; Hemoliance Recombiplastin, Instrumentation Laboratory) was injected via the IVC at a dose of 5 µL/g mouse, using a 27-G needle attached to a 1-mL syringe. Mice were observed for signs of respiratory arrest over 30 minutes. The time of death, defined as the time to onset of respiratory arrest lasting 2 minutes, was recorded. Mice surviving the 30-minute observation period were noted as survivors and euthanized via cervical dislocation.
Statistics
Statistical analysis was performed using Graphpad Prism, version 4.0. Data were checked for normality using the D'Agostino and Pearson omnibus normality test before further statistical analysis. Mouse weight, plasma TAT complexes, and liver lesion burden were compared using the Student t test. Data obtained in CAT assays was compared by 1-way analysis of variance with the Bonferroni posttest. For survival studies, χ2 analysis was performed using the log-rank test. All data are presented as the mean ± standard deviation, unless otherwise stated. For all tests, P < .05 was considered statistically significant.
Study approval
The Institutional Animal Care and Use Committee of the Medical College of Wisconsin approved all animal experiments performed in this study.
Results
Tfpi−/− mice are rescued to wean by lack of PAR4 but not lack of PAR3
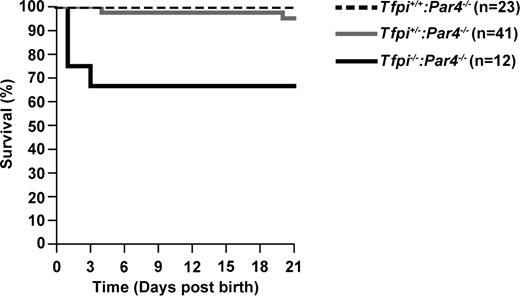
Tfpi+/−mice lacking PAR3 or PAR4 were mated, and the offspring was genotyped at 21 days of age to determine whether decreased platelet responsiveness to thrombin would rescue the embryonic lethal phenotype of TFPI-null mice. Of 108 weanlings obtained from Tfpi+/−:Par3−/− × Tfpi+/−:Par3−/− matings, none were Tfpi−/−:Par3−/−, demonstrating that concomitant lack of PAR3 in the mother and the fetus does not rescue the embryonic lethality of Tfpi−/− mice (Table 1). In contrast, of 70 weanlings obtained from Tfpi+/−:Par4−/− × Tfpi+/−:Par4−/− matings, 8 (45.6% of expected) were Tfpi−/−:Par4−/− (Table 2). In addition to these 8 Tfpi−/−:Par4−/− weanlings, 4 Tfpi−/−:Par4−/− and 2 Tfpi+/−:Par4−/− pups were discovered dead or partially consumed. From the pattern of early perinatal survival (Figure 1), it can be inferred that ≥60% of the Tfpi−/−:Par4−/− mice survive embryogenesis. Of the adult Tfpi−/−:Par4−/− mice not euthanized for experiments, 22 of 23 lived to >8 months of age without overt signs of thrombosis.
TFPI genotype of weanlings from Tfpi+/−:Par3−/− × Tfpi+/−:Par3−/− mating
| TFPI genotype . | Number (n = 108) . | Observed (%) . | Expected (%) . | Observed/expected (%) . |
|---|---|---|---|---|
| Tfpi+/+ | 34 | 31.5 | 25 | 126 |
| Tfpi+/− | 74 | 68.5 | 50 | 137 |
| Tfpi−/− | 0 | 0 | 25 | 0 |
| TFPI genotype . | Number (n = 108) . | Observed (%) . | Expected (%) . | Observed/expected (%) . |
|---|---|---|---|---|
| Tfpi+/+ | 34 | 31.5 | 25 | 126 |
| Tfpi+/− | 74 | 68.5 | 50 | 137 |
| Tfpi−/− | 0 | 0 | 25 | 0 |
TFPI genotype of weanlings from Tfpi+/−:Par4−/− × Tfpi+/−:Par4−/− mating
| TFPI genotype . | Number (n = 70) . | Observed (%) . | Expected (%) . | Observed/expected (%) . |
|---|---|---|---|---|
| Tfpi+/+ | 23 | 32.9 | 25 | 131.6 |
| Tfpi+/− | 39 | 55.7 | 50 | 111.4 |
| Tfpi−/− | 8 | 11.4 | 25 | 45.6 |
| TFPI genotype . | Number (n = 70) . | Observed (%) . | Expected (%) . | Observed/expected (%) . |
|---|---|---|---|---|
| Tfpi+/+ | 23 | 32.9 | 25 | 131.6 |
| Tfpi+/− | 39 | 55.7 | 50 | 111.4 |
| Tfpi−/− | 8 | 11.4 | 25 | 45.6 |
Survival of Tfpi+/+:Par4−/−, Tfpi+/−:Par4−/−, and Tfpi−/−:Par4−/− pups from birth to wean. Litters were observed over the first 21 days of life. Dead pups were genotyped. Pups surviving through 21 days were weaned and genotyped. Shown is the percentage of survivors for each genotype over time.
Survival of Tfpi+/+:Par4−/−, Tfpi+/−:Par4−/−, and Tfpi−/−:Par4−/− pups from birth to wean. Litters were observed over the first 21 days of life. Dead pups were genotyped. Pups surviving through 21 days were weaned and genotyped. Shown is the percentage of survivors for each genotype over time.
Tfpi−/−:Par4−/− mice are fertile
Breeding studies were performed using Tfpi−/−:Par4−/− male (n = 2) or female (n = 4) mice mated to wild-type or Tfpi+/−:Par4−/− mice. Tfpi−/−:Par4−/− mice of either sex successfully produced litters. In addition, a Tfpi−/−:Par4−/− breeding pair successfully produced 2 litters, although with reduced fecundity (7 pups in total while mated for ∼6 months), demonstrating that TFPI is not absolutely required for reproduction. Most of the experimental mice used were from Tfpi−/−:Par4−/− males mated with Tfpi+/−:Par4−/− females, which produced 140 Tfpi−/−:Par4−/− mice from a total of 653 offspring (42.9% of the expected Mendelian frequency; Table 3).
TFPI Genotype of weanlings observed from Tfpi−/−:Par4−/− × Tfpi+/−:Par4−/− mating
| TFPI genotype . | Number (n = 653) . | Observed (%) . | Expected (%) . | Observed/expected (%) . |
|---|---|---|---|---|
| Tfpi+/− | 513 | 78.6 | 50 | 157.1 |
| Tfpi−/− | 140 | 21.4 | 50 | 42.9 |
| TFPI genotype . | Number (n = 653) . | Observed (%) . | Expected (%) . | Observed/expected (%) . |
|---|---|---|---|---|
| Tfpi+/− | 513 | 78.6 | 50 | 157.1 |
| Tfpi−/− | 140 | 21.4 | 50 | 42.9 |
Tfpi−/−:Par4−/− mice are small and may have short and/or curly tails
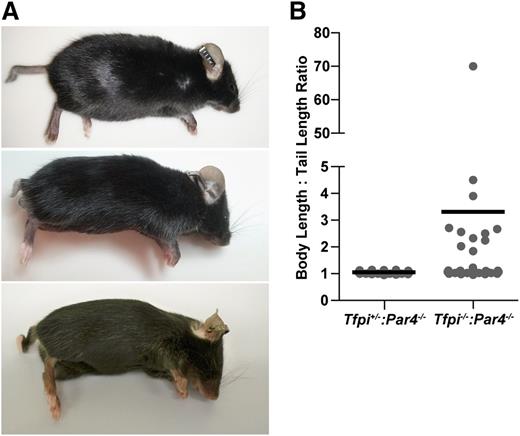
Tfpi−/−:Par4−/− mice weighed slightly less than their Tfpi+/−:Par4-/- littermates at 8 weeks of age (21.8 ± 2.5 g, n = 32, vs 23.1 ± 1.9 g, n = 28; P < .05). A more striking physical phenotype of the Tfpi−/−:Par4−/− mice was that ∼30% were born with a short tail, typically varying from one half to one fourth the length of a normal tail (Figure 2). The tail was completely absent in 1 mouse. All short tails were present at birth and did not shorten further over time. Some Tfpi−/−:Par4−/− mice also had curly or kinked tails (Figure 2A). Tfpi−/−:Par4−/− mice with normal tail length maintained it for their lifetime.
Some Tfpi−/−:Par4−/− mice have short, kinked, and/or curly tails. (A) Representative photos of short tailed Tfpi−/−:Par4−/− mice. (B) The body length to tail length ratio of Tfpi−/−:Par4−/− (n = 38) and Tfpi+/−:Par4−/− (n = 35) littermate male mice was determined at 8 weeks of age. An increased ratio, because of decreased tail length, was observed in 11 (29%) Tfpi−/−:Par4−/− mice. Data points represent ratios of individual mice and black lines the average ratio.
Some Tfpi−/−:Par4−/− mice have short, kinked, and/or curly tails. (A) Representative photos of short tailed Tfpi−/−:Par4−/− mice. (B) The body length to tail length ratio of Tfpi−/−:Par4−/− (n = 38) and Tfpi+/−:Par4−/− (n = 35) littermate male mice was determined at 8 weeks of age. An increased ratio, because of decreased tail length, was observed in 11 (29%) Tfpi−/−:Par4−/− mice. Data points represent ratios of individual mice and black lines the average ratio.
Tfpi−/−:Par4−/− mice have sterile inflammatory lesions with associated fibrin deposition in the liver
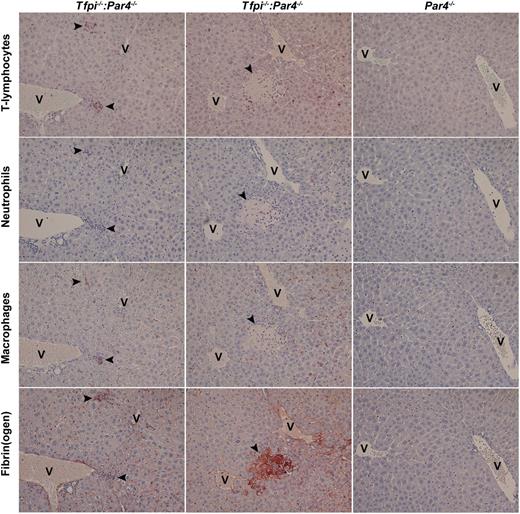
Histologic analysis was performed on the major organs from Tfpi−/−:Par4−/− mice, including the brain, heart, lungs, thymus, liver, spleen, and kidneys. Sterile infiltrates of inflammatory cells were present in all Tfpi−/−:Par4−/− mouse livers examined (Figure 3). Two lesion types were observed. Smaller lesions, containing predominantly CD3+ lymphocytes and macrophages with fibrin(ogen) deposition, were most common (Figure 3, column 1). Rarely, larger, necrotic, fibrin(ogen)-rich lesions, containing neutrophils and surrounded by CD3+ lymphocytes, were also observed (Figure 3, column 2). Liver of Tfpi−/−:Par4−/− mice contained approximately sixfold more lesions per square millimeter of tissue compared with Par4−/− mice (0.206 ± 0.031 vs 0.035 ± 0.039 lesions/mm2, respectively; P < .005). CBCs in 8- to 12-week-old Tfpi−/−:Par4−/− mice were not different from CBCs in Par4−/− aged-matched control mice (supplemental Table 1 available on the Blood Web site), indicating the absence of a systemic inflammatory condition. The remaining organs were histologically normal when examined after trichrome staining.
Sterile inflammatory infiltrates are present in the liver of Tfpi−/−:Par4−/− mice. Livers were isolated from Tfpi−/−:Par4−/− mice (first and second columns) and Par4−/− mice (third column) and processed for immunohistochemistry. Serial sections were stained for T lymphocytes (first row, CD3-positive cells), neutrophils (second row, Ly6B.2-positive cells), macrophages (third row; F4/80-positive cells), and fibrin(ogen) (fourth row). Two types of lesions were observed in Tfpi−/−:Par4−/− mice: smaller lesions containing predominantly CD3+ cells and macrophages that were associated with fibrin deposition (first column) and large, necrotic, fibrin-rich lesions containing neutrophils that were surrounded by CD3+ cells (second column). Staining of tissue from Par4−/− mice (third column) served as a control. In each image, lesions are indicated with arrowheads, and the central vein is indicated with the letter V. Slides were examined at room temperature using Olympus 10X UPlanFI and ×20 Plan objectives lenses mounted to a Olympus BX50 microscope. Coverslips were mounted using xylene-compatible mounting medium (Dako). Images were captured with a Nikon DS-Fi1 digital microscope camera and Nikon NIS Elements software (version 2.1).
Sterile inflammatory infiltrates are present in the liver of Tfpi−/−:Par4−/− mice. Livers were isolated from Tfpi−/−:Par4−/− mice (first and second columns) and Par4−/− mice (third column) and processed for immunohistochemistry. Serial sections were stained for T lymphocytes (first row, CD3-positive cells), neutrophils (second row, Ly6B.2-positive cells), macrophages (third row; F4/80-positive cells), and fibrin(ogen) (fourth row). Two types of lesions were observed in Tfpi−/−:Par4−/− mice: smaller lesions containing predominantly CD3+ cells and macrophages that were associated with fibrin deposition (first column) and large, necrotic, fibrin-rich lesions containing neutrophils that were surrounded by CD3+ cells (second column). Staining of tissue from Par4−/− mice (third column) served as a control. In each image, lesions are indicated with arrowheads, and the central vein is indicated with the letter V. Slides were examined at room temperature using Olympus 10X UPlanFI and ×20 Plan objectives lenses mounted to a Olympus BX50 microscope. Coverslips were mounted using xylene-compatible mounting medium (Dako). Images were captured with a Nikon DS-Fi1 digital microscope camera and Nikon NIS Elements software (version 2.1).
Tfpi−/−:Par4−/− mice have elevated plasma TAT levels
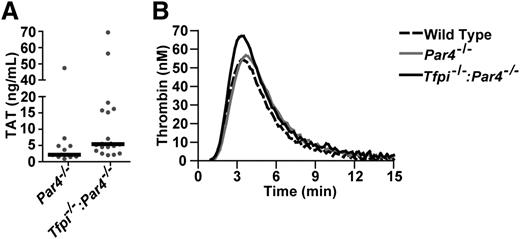
Plasma TAT complexes were increased approximately twofold in Tfpi−/−:Par4−/− mice compared with Par4−/− mice, suggesting these mice have a systemic activation of coagulation when maintained under standard husbandry conditions (Figure 4A). Plasma TAT concentration was not different between young (8-12 weeks) and old (>8 months) mice of the same genotype (P > .05). Plasma d-dimer was not detected in mice of any age or genotype.
Tfpi−/−:Par4−/− mice have enhanced thrombin generation in vivo and in vitro. (A) TAT complex in plasma is elevated in Tfpi−/−:Par4−/− mice compared with Par4−/− mice (13.4 ± 18.1 ng/mL, n = 20 vs 6.7 ± 13.0 ng/mL, n = 12; mean ± standard deviation; P = .014). (B) Real-time thrombin generation in PPP from wild-type, Par4−/−, and Tfpi−/−:Par4−/− mice was assessed by calibrated automated thrombography. Lines represent the mean thrombin generated in pooled samples (n = 2 for each genotype) from 3 separate experiments using a total of 6 mice for each genotype.
Tfpi−/−:Par4−/− mice have enhanced thrombin generation in vivo and in vitro. (A) TAT complex in plasma is elevated in Tfpi−/−:Par4−/− mice compared with Par4−/− mice (13.4 ± 18.1 ng/mL, n = 20 vs 6.7 ± 13.0 ng/mL, n = 12; mean ± standard deviation; P = .014). (B) Real-time thrombin generation in PPP from wild-type, Par4−/−, and Tfpi−/−:Par4−/− mice was assessed by calibrated automated thrombography. Lines represent the mean thrombin generated in pooled samples (n = 2 for each genotype) from 3 separate experiments using a total of 6 mice for each genotype.
Lack of plasma TFPI in Tfpi−/−:Par4−/− mice contributes to increased thrombin generation
TF-initiated CAT assays were performed using PPP from wild-type, Par4−/−, and Tfpi−/−:Par4−/− mice (Figure 4B). Thrombin generation profiles in wild-type and Par4−/− PPP were nearly identical, consistent with PAR4 having no effect on blood coagulation in plasma systems. The lack of functional TFPI in PPP obtained from Tfpi−/−:Par4−/− mice resulted in faster (P < .01) and augmented (P < .01) peak thrombin generation (Figure 4; supplemental Table 2).
TFPI deficiency does not affect in vivo thrombus formation in Par4−/− mice
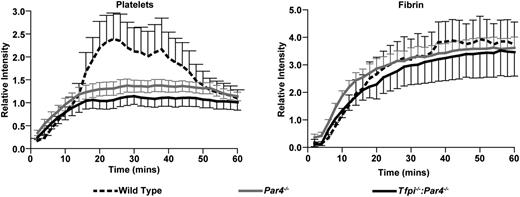
To examine the effect of combined TFPI and PAR4 deletion on intravascular thrombus formation, electrolytic injury was used to trigger femoral vein thrombosis in wild-type, Par4−/−, and Tfpi−/−:Par4−/− mice. Consistent with a previous study that used a laser-induced cremaster arteriole injury model,14 Par4−/− mice had decreased platelet accumulation but normal fibrin formation compared with wild-type mice (Figure 5). Tfpi−/−:Par4−/− mice also had decreased platelet accumulation and normal fibrin formation (Figure 5), demonstrating that the absence of TFPI activity does not alter thrombus formation in mice lacking PAR4 in this model of vascular injury.
Lack of TFPI does not affect the dynamics of clot formation in Par4−/− mice. Thrombus formation in the femoral vein was induced by electrolytic injury. For each genotype, 2 thrombi were formed in each of 3 mice, 1 in each femoral vein, totaling 6 thrombi analyzed. Lines represent the mean and error bars the standard deviation of the relative intensities for accumulation of rhodamine 6G-labeled platelets and AlexaFluor-647–labeled anti-fibrin. Platelet accumulation is significantly decreased in both Par4−/− (P < .01) and Tfpi−/−:Par4−/− (P < .001) mice compared with wild-type mice. It is not significantly different between Par4−/− and Tfpi−/−:Par4−/− mice (P > .05). Fibrin formation is not different among the genotypes (P > .05 for all comparisons).
Lack of TFPI does not affect the dynamics of clot formation in Par4−/− mice. Thrombus formation in the femoral vein was induced by electrolytic injury. For each genotype, 2 thrombi were formed in each of 3 mice, 1 in each femoral vein, totaling 6 thrombi analyzed. Lines represent the mean and error bars the standard deviation of the relative intensities for accumulation of rhodamine 6G-labeled platelets and AlexaFluor-647–labeled anti-fibrin. Platelet accumulation is significantly decreased in both Par4−/− (P < .01) and Tfpi−/−:Par4−/− (P < .001) mice compared with wild-type mice. It is not significantly different between Par4−/− and Tfpi−/−:Par4−/− mice (P > .05). Fibrin formation is not different among the genotypes (P > .05 for all comparisons).
Tfpi−/−:Par4−/− mice are more susceptible to TF-induced pulmonary embolism than Par4−/− mice
A pulmonary embolism model initiated by intravascular injection of TF was used to further examine the effect of combined TFPI and PAR4 deletion on intravascular thrombus formation. Eleven of 12 wild-type mice died from respiratory arrest within 5 minutes. In contrast, 12 of 12 Par4−/− mice survived the entire 30-minute observation period (Figure 6), confirming previous studies demonstrating that the lack of PAR4 protects mice from TF-induced pulmonary embolism.16 Two of 6 Tfpi−/−:Par4−/− mice died from respiratory arrest within 5 minutes, whereas the remaining 4 survived the entire 30-minute observation period, demonstrating that the absence of TFPI activity partially overrides the protective effect of PAR4 deficiency in this pulmonary embolism model.
Lack of TFPI increases the susceptibility to thrombosis of Par4−/− mice in a TF-induced pulmonary embolism model. The time to respiratory arrest after injection of TF was measured. Shown is the percentage of survivors over time. The fraction of surviving Par4−/− mice is greater than surviving Tfpi−/−:Par4−/− mice (P = .0363), and the fraction of both Par4−/− and Tfpi−/−:Par4−/− surviving mice is greater than wild-type mice (P < .0001 and P = .0319, respectively).
Lack of TFPI increases the susceptibility to thrombosis of Par4−/− mice in a TF-induced pulmonary embolism model. The time to respiratory arrest after injection of TF was measured. Shown is the percentage of survivors over time. The fraction of surviving Par4−/− mice is greater than surviving Tfpi−/−:Par4−/− mice (P = .0363), and the fraction of both Par4−/− and Tfpi−/−:Par4−/− surviving mice is greater than wild-type mice (P < .0001 and P = .0319, respectively).
Discussion
Tfpi−/−:Par4−/− mice are the first adult mice described to lack TFPI but have unaltered TF expression. Their survival demonstrates that TFPI modulates thrombin-dependent platelet activation in a manner required for murine embryonic survival. Although Par3−/− and Par4−/− mice are equally protected from FeCl3-induced thrombosis of the mesenteric arteriole or TF-induced pulmonary embolism,16 only concomitant deficiency of PAR4 rescued the embryonic lethal phenotype of Tfpi−/− embryos. Adult Tfpi−/−:Par4−/− mice, although slightly smaller than littermates, are fertile and have a normal life expectancy. Approximately one third have short tails. Tfpi−/−:Par4−/− mice do not have overt signs of thrombosis, but they do have a systemic prothrombotic state evidenced by elevated plasma TAT complexes and isolated sterile inflammatory foci in the liver that are associated with fibrin deposits. Accordingly, Tfpi−/−:Par4−/− mice have increased susceptibility to TF-induced pulmonary embolism compared with Par4−/− mice. The findings identify TFPI as an essential, indirect inhibitor of PAR4 activation during embryogenesis. They also highlight a role for TFPI in adult animals in dampening intravascular procoagulant stimuli that lead to thrombin generation, even in the absence of thrombin-mediated platelet activation.
Tfpi−/− mice die at 2 stages during embryonic development.4,6 On a C57Bl/6 background, ∼30% die between E9.5 and E11.5 due to yolk sac hemorrhage and circulatory collapse, whereas the remaining 70% die from an apparent consumptive coagulopathy before birth.4,6 The rescue of this embryonic lethality by concomitant PAR4 deficiency, but not concomitant PAR3 deficiency, suggests that Tfpi−/− embryos produce thrombin at a concentration sufficient to activate platelet PAR4 in the absence of PAR3 and that this platelet activation contributes to their demise. PAR4 is also expressed on endothelium;17 however, it is unlikely that this contributes to the embryonic rescue as bone marrow transplantation studies have found that the protection from thrombosis provided by PAR4 deficiency is accounted for entirely by the lack of thrombin activation of platelets.18 In addition, thrombin signaling in mouse endothelial cells occurs primarily through PAR1.17 This suggests that the lack of platelet PAR4 dampens development of the later stage consumptive coagulopathy, allowing birth of Tfpi−/− embryos that survive the early stage yolk sac hemorrhage.
Male and female Tfpi−/−:Par4−/− mice are fertile. Male Tfpi+/−:Par4−/− and Tfpi−/−:Par4−/− mice produce Tfpi−/−:Par4−/− offspring at a similar Mendelian frequency when mated to Tfpi+/−:Par4−/− female mice, suggesting that the lack of TFPI in the male has little effect on reproductive efficiency. In contrast, fecundity is markedly reduced when Tfpi−/−:Par4−/− males are mated with Tfpi−/−:Par4−/− females. TFPI is produced by endothelial19 and trophoblast20 cells of the placenta, and placental TFPI prevents fibrin deposition and apoptosis that contributes to fetal death.6 Findings presented here are consistent with those suggesting that maternal TFPI within the placenta is important for successful pregnancy outcome.21
The most striking physical characteristic of Tfpi−/−:Par4−/− mice is the presence of short, curly, or kinked tails. This phenotype was also observed in the originally characterized Tfpi−/− embryos, where they were associated with hemorrhage within the tail.4 Deformed tails were not observed in Par4−/− mice, suggesting that they are caused by TFPI deficiency in the Tfpi−/−:Par4−/− mice. Of interest is that Tfpi−/−:TFlow mice have normal tail length,6 suggesting that the observed tail deformities may be caused by unfettered TF activity that is normally inhibited by TFPI. Importantly, the Tfpi−/−:Par4−/− mice born with normal tails did not develop deformed tails over their lifespan, as might be expected if they resulted from vascular thrombosis. Signaling through PAR1 and PAR2 contributes to neural tube closure, and adult Par1−/−:Par2−/− mice have curly tails.22 TF indirectly signals through PAR1 by initiating thrombin production, and TFPIβ, an alternatively spliced form of TFPI on endothelium,23 potently inhibits this activity.24 TF-fVIIa can also signal through PAR2, either directly or indirectly by promoting fXa production.25 Thus, it is tempting to speculate that tail defects in the Tfpi−/−:Par4−/− mice develop as a consequence of altered TF-dependent PAR1 and/or PAR2 signaling. Alternatively, the tail defects could result from the action of thrombin on its other targets, such as GP1b or fibrinogen. Further studies are required to explore these possibilities.
Adult Tfpi−/−:Par4−/− mice do not display overt thrombosis. However, they exhibit augmented thrombin production in plasma CAT assays and elevated plasma TAT levels, implying the presence of systemic activation of coagulation. Thus, it appears that TFPI dampens intravascular prothrombotic stimuli that occur under standard husbandry conditions in Par4−/− mice. Because Par4−/− platelets are unresponsive to thrombin, this anticoagulant activity may be mediated by endothelial TFPI. A role for endothelial TFPI in dampening intravascular prothrombotic stimuli also is supported by the recent findings of elevated plasma d-dimer and prothrombin fragment 1+2 concentration in human subjects treated with intravenous or subcutaneous anti-TFPI antibody.26
The presence of a prothrombotic condition in the Tfpi−/−:Par4−/− mice is further demonstrated by the presence of focal, mixed inflammatory cell infiltrates along with fibrin(ogen) deposition in the livers of Tfpi−/−:Par4−/− mice that develop under standard husbandry conditions. It is notable that very similar lesions were observed in mice with combined TFPI heterozygosity and partial thrombomodulin deficiency (Tfpi+/−:Thbdpro/pro),21 and liver-specific fibrin deposition was noted in the original description of the Tfpi−/− embryos.4 Taken together, these data suggest that TFPI anticoagulant function is particularly important to regulate coagulation in the liver microenvironment, where the unique cellular composition and fenestrated endothelium demand strict control of hemostasis.
Par4−/− mice have decreased platelet accumulation but unaltered fibrin deposition in a cremaster arteriole laser-injury model,14 suggesting that active coagulation complexes may form locally on cellular surfaces distinct from the platelet. Indeed, Ivanciu et al recently demonstrated that prothrombinase can assemble on endothelium.27 In a large-vessel venous electrolytic injury model, mice lacking hematopoietic cell TFPI produce thrombi with increased platelet accumulation but unaltered fibrin deposition.13 Here, we used the same electrolytic injury model to find that Tfpi−/−:Par4−/− mice have decreased platelet accumulation and unaltered fibrin deposition at the injury site, mirroring the findings obtained in Par4−/− mice. Thus, the lack of PAR4 renders the anticoagulant activity of platelet TFPI unnecessary in this model. Tfpi−/−:Par4−/− mice also completely lack endothelial and plasma TFPI activity. Mice lacking endothelial TFPI have a shorter time to occlusion in a FeCl3-induced thrombosis model,28 and the data presented here suggest that TFPI regulates TF-dependent, platelet-independent clot formation in a TF-induced pulmonary embolism model. Therefore, it was somewhat unexpected to find that fibrin generation was not different between wild-type and Tfpi−/−:Par4−/− mice in the electrolytic injury model. It may be that TF exposure at the injury site overwhelms endothelial TFPI, effectively making this model insensitive to changes in endothelial TFPI, or that other anticoagulants, such as antithrombin and those in the protein C/S system, compensate for the lack of TFPI.
To examine how the lack of TFPI alters TF-initiated coagulation in vivo, we used a pulmonary embolism model, in which clot formation is induced by the injection of TF directly into the vasculature.16 Mice lacking PAR4 are completely protected from respiratory arrest in this model, suggesting that thrombin-dependent platelet activation is a primary cause of thrombus formation. The lack of TFPI in Tfpi−/−:Par4−/− mice partially reversed the protective effect of the lack of PAR4. This suggests that TF-dependent, platelet-independent clot formation (presumably fibrin-rich) may occur in this model, possibly on the surface of activated endothelium or the phospholipid in which the injected TF is embedded, and that this is inhibited by endothelial or plasma TFPI in Par4−/− mice.
The mechanisms whereby TFPI deficiency promotes consumptive coagulopathy and embryonic lethality in mice are not fully understood. Our studies here offer new insight into the cellular basis whereby TFPI inhibits this lethal procoagulant event. Specifically, the partial rescue of this embryonic death by concomitant lack of PAR4 strongly suggests that TFPI functions to dampen thrombin-mediated platelet activation and the ensuing consumptive coagulopathy during murine development. Tfpi−/−:Par4−/− mice surviving to adulthood have a prothrombotic phenotype associated with elevation in plasma TAT concentration and tissue-specific fibrin deposition in the liver, but without overt thrombosis. Collectively, the results suggest that the anticoagulant function of TFPI, mediated through either its ability to inhibit TF or its ability to inhibit early forms of prothrombinase, is essential for dampening of thrombin-mediated platelet activation during embryogenesis and adult life.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
This work was supported by National Institutes of Health National Heart, Lung, and Blood Institute grants HL068835 (to A.E.M.), HL096149 and HL117702 (to S.A.M.), and HL117132 (to H.W.) and National Institute of Environmental Health Sciences grant ES017537 (to J.P.L.). Further research support to A.E.M. was received from Novo Nordisk.
The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Authorship
Contribution: P.E.R.E. and S.A.M. designed and performed experiments and wrote the manuscript; B.C.C. examined in vivo thrombus formation in all mice after electrolytic injury of the femoral vein; J.P.L. performed histological analysis of liver sections; M.Z. aided in the design of mouse breeding strategies and performed all genotyping; and H.W. and A.E.M. designed experiments, interpreted results, and wrote the manuscript.
Conflict-of-interest disclosure: A.E.M. receives research grant support from Novo Nordisk and has received honoraria from Novo Nordisk, Siemens, and Portola. J.P.L. receives research grant support from Boehringer Ingelheim. The other authors declare no competing financial interests.
The current affiliation for P.E.R.E. is School of Biomedical Sciences, Curtin University, Bentley, WA, Australia.
The current affiliation for B.C.C. is Department of Pathology and Laboratory Medicine, University of North Carolina, Chapel Hill, NC.
Correspondence: Alan E. Mast, Blood Research Institute, Blood Center of Wisconsin, PO Box 2178, Milwaukee, WI 53201; e-mail: alan.mast@bcw.edu.
References
Author notes
P.E.R.E. and S.A.M. contributed equally to this work.