Abstract
Acute myeloid leukemia (AML) in older patients presents a notable therapeutic challenge to the clinical hematologist. The clinical biology of AML among patients is highly heterogeneous. Interpatient variations are relevant for prognosis and treatment choice. Outcome of treatment for patients of advanced age is often compromised by comorbid conditions and an enhanced susceptibility to toxicities from therapy. Here we present selected clinical vignettes that highlight distinct representative situations derived from clinical practice. The vignettes are specifically discussed in light of the perspective of treating older patients with leukemia. We review the clinical significance of various cytogenetic and molecular features of the disease, and we examine the various currently available treatment options as well as the emerging prognostic algorithms that may offer guidance in regard to personalized therapy recommendations. The dilemmas in tailoring treatment selection in this category of patients with AML are the central theme in this discussion.
Introduction
The median age of patients with acute myeloid leukemia (AML) is around 70 years.1 Calendar age reflects an absolute value but it ignores the “biological age” that is representative of the physical condition, which may vary considerably among older people of the same age. In any event, although old age is not a feature that defines a disease entity, it is of significant clinical relevance because it confers a profound prognostic impact on disease outcome. Treatment outcome in patients with AML continuously declines with progressively increasing age.2 Some of the key questions for clinical hematologists in daily practice are: (1) Which patient of an older age can receive intensive treatment and not experience prohibitive toxicity? and (2) Even in those who can tolerate such chemotherapy, would disease features make the likelihood of benefit so low that nonintensive therapy would be a better option?
In industrialized societies, the average life expectancy of a person at age 65 years is still approximately 15 to 20 more years, which underscores the considerable lifetime that can be gained if AML at that age could be cured. Specific clinical trials have been dedicated to the older-aged segment of patients with the objective of improving their treatment outcome. Clinical trials for practical reasons have usually applied age cutoffs above ages 60 to 70 years as operational definitions for older patients. Enrollment in such trials usually implies that the investigator assumes that the patient will tolerate intensive chemotherapy. On average, a significant proportion of about 50% to 60% of patients will successfully attain a complete remission (CR). However, such fairly high rates of good response translate into a 2-year survival of only about 15% to 20%.3 These outcome results have only very modestly improved in the last decade, in particular in patients younger than 75 years.4-6 However, there is a significant individual heterogeneity that likely accounts for a substantial variation in treatment outcome among patients (eg, in relation to leukemia cytogenetics and molecular genetics).3,4 The determinants of success and failure nevertheless remain only partly understood.
These determinants are clearly multifold and include a combination of patient-related and specific disease-related factors.7
Patient-related prognostic factors: Comorbid conditions are more frequent in patients at an older age. These and performance status are among the most critical patient-related factors. Pharmacokinetic and pharmacodynamic changes result in decreased drug clearance, causing extended prolonged exposure to chemotherapeutics. The decreased immune competence of elderly patients results in less tolerability of infections. This all influences outcome because of increased toxicity of the treatment. In addition, psychosocial factors like cognitive decline, social isolation, and, often, lack of caretakers are factors that influence the outcome. The patient-related determinants may create hurdles to sufficiently deliver dose-intensive chemotherapy safely.
Disease-related prognostic factors: Higher frequencies of adverse cytogenetics and unfavorable molecular aberrations, multidrug-resistant abilities of the leukemia cells to expel the chemotherapeutics that had initially entered the cell, and antecedent hematologic disorders are all more common among the aging population. They correlate markedly with treatment failure (ie, primary resistance and relapse after induction therapy).4 A distinct gene-expression profile noted for older compared with younger patients supports a molecular basis for poor outcomes in elderly patients.8,9
In this article we present 4 selected clinical vignettes that highlight our treatment approach in clinical practice in light of the biology of the disease, and we discuss some of the common practical issues and dilemmas we have encountered in the therapeutic management of older patients with AML.
Which patients qualify as candidates for intensive treatment?
Population data from the Swedish Acute Leukemia Registry suggest that the majority of older patients should be regarded as candidates for intensive chemotherapy. These national registry data show that, in general, older patients with AML fare markedly better receiving intensive chemotherapy than palliative treatment. Performance status rather than age in the strict sense is predictive of early mortality.10,11 Yet a particular proportion of AML patients will not tolerate the use of intensive chemotherapy. Those patients may be offered demethylating agents (eg, decitabine, azacitidine) as a less-intensive modality of treatment.12 The recognition of patients who may more likely benefit from an intensive treatment approach should ideally be based on baseline assessments. It remains a challenge both to identify those patients before the start of treatment and to define their features. A variety of composite multifactorial risk algorithms have been proposed in which patient-specific factors (eg, performance, comorbidity scores) together with disease-specific factors (eg, [cyto]genetics, white blood cell counts, percent marrow blast count, secondary leukemias) have been taken into account to predict treatment effectiveness and lend support to a documented choice between intensive treatment and various other treatment possibilities.13-15 An inherent limitation of all of these risk algorithms is that they have been derived from data of a patient population that had already been selected for intensive treatment, and thus they do not reflect the average real world of older patients with AML who normally present in our consulting room. For instance, in the French ALFA 9803 trial (elderly AML trial), no more than 5% of the included 416 patients had 3 or more comorbidities.16 The Hematopoietic Cell Transplantation Comorbidity Index (HCT-CI) scoring system, which is based only on comorbidities and was developed for estimating transplant-related mortality, has also been evaluated for patients with AML treated with intensive chemotherapy. It appears of some use for selecting patients for intensive chemotherapy because it provides a reflection of their concomitant diseases.17 For example, a relatively high HCT-CI score of ≥3 is associated with an early death rate that may be as high as 30%. More recently, another prediction model for early mortality after induction therapy that is based on age, performance status, and platelet count has been introduced and validated in independent cohorts of patients.18 Geriatric assessments with a focus on cognitive and physical functions have also been demonstrated to express predictive value for outcome of induction treatment in elderly patients with AML.19,20 Nevertheless, the reality of clinical trials implies that a certain proportion of older patients will always be excluded from intensive chemotherapy trial participation irrespective of these assessments because of their inability to meet the eligibility criteria.
As yet, none of these risk algorithms has become widely accepted. However, the decreased rate of treatment-related mortality in intensively-treated patients recently described could be partly explained by a better selection of patients suitable for this intensive therapy.21 Additional research on the developments of measurements that are solidly validated and (preferably) quite easily applicable in clinical practice is ongoing.
Conversely, particular disease-specific biological characteristics of AML may be associated with such a poor outcome that even though patients may be considered medically fit, they will not likely benefit from intensive treatment and therefore should perhaps rather be offered a less intensive or investigational approach. The biological characteristics of these high-risk AMLs include, for example, overexpression of the oncogene EVI-1,22,23 ASXL1 gene mutations, biallelic FLT3-ITDs, p53 gene mutations,24 and complex and/or monosomal karyotypes.3,25 We would consider intensive treatment in such patients only in case an allogeneic hematopoietic stem cell transplantation (HSCT) is foreseen as a possible prospective option after attainment of CR.
Patient 1: AML with unfavorable features in a 68-year-old man
This 68-year-old man was diagnosed with AML with a relatively low blast count (marrow blast infiltrate 21%) with normocytic anemia (Hgb 5.5 mmol/L), a white blood cell count (WBC) of 3.5 × 109/L, and a severe thrombocytopenia of 25 × 109/L. Cytologic examination of the marrow showed dysplastic signs in various cell lineages and an abnormal karyotype with 45,XY, 1p-, −17, 17q+, 20q+, −22, + mar1(4). Thus, the patient presented with a notably unfavorable type of AML that included a complex karyotype (≥3 clonal cytogenetic aberrations) and a monosomal karyotype (multiple monosomies and also additional structural aberrations).25 Molecular analysis revealed high EVI1 transcript expression and −17 and 17q+, 2 other adverse signs.22,23 He exhibited good physical performance but, as is quite common among older patients, he had multiple comorbid conditions including prior surgery for benign prostate hypertrophy, chronic obstructive pulmonary disease, atrial fibrillation, and hypertension. Two years prior he had received a coronary stent for angina pectoris and coronary stenosis. His left ventricular function, however, was normal. According to our general approach, remission-induction chemotherapy was undertaken with the intent to lead the patient to a CR and subsequently try to progress to allogeneic HSCT. The first remission-induction cycle with daunorubicin (45 mg/m2 on each of 3 days) and cytarabine (Ara-C) (200 mg/m2 ci, 7 days) was complicated by fever and coagulase-negative Staphylococcus septicemia. A CR with incomplete platelet recovery (platelet count of 45 000) (CRi) ensued. Subsequently, a consolidation cycle with intermediate-dose Ara-C (1000 mg/m2 twice daily IV over 6 hours on days 1-6) initiated after an interval of 32 days after the start of induction cycle I was complicated by considerable gastrointestinal toxicity and diarrhea. At 3 months from diagnosis, he presented with severe pain in his arms and back, which originated from spondylodiscitis (C5-C6) caused by an infection with Staphylococcus epidermidis and Citrobacter freundii, which required IV antibiotic treatment. Thus, because of the latter intercurrent medical problems, additional antileukemia treatment in this older patient had to be postponed. Meanwhile, the CRi continued. In the absence of an available HLA-identical family donor, an unrelated donor search had yielded a 12/12 HLA-matched donor. At 5.5 months after the start of treatment at age 69, our patient received an allograft after a reduced-intensity conditioning regimen with fludarabine and 2Gy total body irradiation (TBI) and postransplant immunoprophylaxis with mycophenolate and cyclosporine. There was early engraftment with full hematologic recovery within 2 weeks, and complete donor chimerism ensued with no apparent signs of graft-versus-host disease. Mycophenolate was discontinued at 3 months and cyclosporin discontinued at 6 months. Currently, the patient survives in good performance and remains disease-free at 24 months after diagnosis.
Comments about patient 1
This patient illustrates that in a fit elderly patient with AML, even with unfavorable risk characteristics and various comorbid conditions, it may be useful to embark on a treatment plan with curative intent with intensive chemotherapy followed by allogeneic HSCT. Clinicians with a treatment goal in mind that has been defined in advance should obviously be prepared to adjust their plan according to the course of medical developments. There is no basis for an absolute a priori fatalism, not even when there are various unfavorable signs, although a favorable outcome as described here will be relatively uncommon. This leukemia carried 3 types of adverse genomic abnormalities that each define poor outcome.21 The AML exhibited chromosomal abnormalities additional to a single monosomy and thus fulfilled the criterion of a monosomal karyotype,25,26 and the AML also exhibited high expression of the oncogene EVI1, a known unfavorable feature in younger adults with AML.22 Furthermore, the loss of chromosome 17 is also a high-risk prognostic marker in AML. A recent analysis from an international study consortium has confirmed the generally poor outcome of patients with AML with various abnormalities that involve 17p and include −17, so-called “abn(17p),” where the P53 gene is located.27 If a direction toward intensive chemotherapy is chosen, the older patient deserves adequate dose-intensified chemotherapy rather than a chemotherapy regimen with unsubstantiated dose-level reductions.3,28 Physicians currently also often deviate to a default of the use of demethylating agents, but it should be considered that a large amount of data are available addressing intensive chemotherapy. Intensive chemotherapy data indicate a substantial probability of CR and prolonged survival in responders, whereas the accumulating evidence arguing in favor of demethylating agents in AML with high blast count is still limited. However, the relation between CR and overall survival (OS) in patients treated with hypomethylating agents as well as mitigated chemotherapy may be less clear-cut than it is for intensive chemotherapy. Recent Medical Research Council trials with either clofarabine or gemtuzumab as adjuncts to low-dose Ara-C (LDAC) revealed a higher CR rate but no better OS.29,30 Nevertheless, it is generally accepted that achieving CR is a first necessary positive step on the way to improved outcome. Survival beyond 3 years is unlikely if CR has not been achieved.31 Whenever possible, it may make sense to try and lead these patients to allogeneic HSCT in a way that is similar to the approach that is pursued in younger and middle-aged adults.4,32,33 In a fraction of patients, a CR may be achieved with demethylating agents. The use of these agents is associated with less toxicity and may also lead the way toward allogeneic HSCT rescue treatment.12 In recent years, allogeneic HSCT has been more commonly applied at higher ages because long-term data indicate that allogeneic HSCT after reduced-intensity conditioning is not only associated with reduced mortality but also exhibits significant antileukemic effectiveness in a range that is very similar to that of ablative allogeneic HSCT.34 Thus, the options in older patients with AML for consolidation with allogeneic HSCT have increased in recent years. Clearly, the decisions regarding treatment choices, in particular in patients of very advanced age (eg, >75 years), deserve a careful discussion about the alternative options (ie, type of remission induction therapy, level of dose intensity in relation to CR probability and expected toxicities, leukemia-specific prognostic risk, consideration of the option of HSCT for consolidation) according to a tailored, individualized approach.
Patient 2: A 64-year-old man with AML with a favorable molecular genotype
A 64-year-old man was admitted for fever. The hematology laboratory had reported a reduced platelet count of 52 × 109/L and a WBC of 9.3 × 109/L with 90% blasts in the differential and a normal hemoglobin value. The marrow showed 87% infiltrate with blasts that were almost entirely Sudan Black–positive (99% of blasts). Cytogenetic examination of 21 metaphases exhibited normal 46,XY cytogenetics, but molecular analysis revealed biallelic mutations of both CEBPA or CEBP-α genes (ie, mutations in both alleles of the gene for the transcription factor CAAT-binding protein). The immunophenotype of the blasts was consistent with AML. Thus, the patient was diagnosed with AML with mutated CEBPA (an entity in the classification WHO2008). The CEBPA biallelic mutant, a recurrent gene abnormality, designates this a leukemia of favorable risk.27,28 The patient began treatment with an induction regimen consisting of Ara-C 200 mg/m2 daily for 7 days and idarubicin 12 mg/m2 on each of days 1, 2, and 3, and he promptly achieved CR. After 6 weeks, he received consolidation chemotherapy that included amsacrine and intermediate-dose Ara-C (1000 mg/m2 every 12 hours for 6 days), and after 12 weeks he received a final cycle of consolidation with mitoxantrone and etoposide. The subsequent course was uneventful, but 2.5 years after his initial diagnosis, 6% circulating blasts reappeared in the blood and the marrow showed an infiltrate with 11% blasts. The same CEBPA mutations were noted and chromosomal examination now also showed cytogenetic evolution of the disease with newly acquired cytogenetic abnormalities (ie, 46,XY,del(11)(q13q23), idic(17)(p11) [5]/46,XY [15]). Because of the comparatively long interval between the emerging relapse and diagnosis, it was decided to reinduce the patient with an anthracyclin–Ara-C regimen, and the patient attained a second CR within 5 weeks with restoration of normal cytogenetics and molecular genetics. There was no matched family donor, and the patient (then at age 66 years) proceeded to an unrelated HLA-matched HSCT according a protocol similar to that in patient 1. Currently at 18 months after relapse, the patient continues in unmaintained second CR.
Comments about patient 2
This patient has an estimated prognosis that markedly contrasts from that of patient 1. He has AML with a relatively favorable genotype (ie, a normal karyotype with biallelic mutant CEBPA). These “favorable” leukemias show an average probability of ∼70% survival at 3 years among adults younger than 60 years of age.35,36 In addition, elderly patients with a favorable risk profile have a distinctly better outcome compared with the other cytogenetic and molecular risk groups (Figure 1).3,37 In this regard, it is of note that in younger adults with favorable genotypes, OS is similar between those who receive an allogeneic HSCT in first CR (CR1) and those who do not, so that in the good-risk patients, the option of an allogeneic HSCT is usually reserved in case leukemia recurs.33,38,39 Also, autologous stem cell transplant applied in CR1 reduces the probability of relapse, with similar OS results.39 The probability of attaining a second CR in AML with a favorable genetic profile is comparatively high, which enhances the feasibility of salvage with an allotransplant in case of relapse.40 This probably holds similarly for patients with any favorable genotype, in particular the core-binding AMLs, AML with NPM1 mut/FLT3-ITD neg (nucleophosmin-1 gene mutation and absence of fms-like tyrosine kinase gene internal tandem duplications),39 and AML with biallelic CEBPA mutants.38 Although autologous HSCT and allogeneic HSCT in younger and middle-aged adults with favorable subtypes of AML offer similar probabilities of OS, it is unknown whether these relationships can be extrapolated and be maintained the same as in older patients. Thus, in the patient presented here, there are 2 defendable therapeutic strategies for consolidation in CR1 (ie, to apply chemotherapy or autologous HSCT and keep the option of an allogeneic HSCT as a backup for relapse, or to immediately proceed to an allogeneic HSCT). The downside of an allogeneic HSCT in CR1 obviously involves the risks of alloimmune-mediated complications and greater mortality; but alternately, completion of the entire treatment within a one-time, concentrated, intensive approach with a reduced risk of recurrence might be seen as an advantage. In any event, there is no compelling argument for guiding patients with low-risk AML to an early allogeneic HSCT in CR1. In the patient presented here, based on the knowledge available at the time, the choice was made in favor of chemotherapy as first-line treatment without HSCT. Fortunately, after relapse, our patient readily entered a second CR, which is quite characteristic for patients with favorable cytogenetic or molecular features.41
Overall survival according to cytogenetic risk in patients above 60 years. Data derived from the HOVON/SAKK study (HOVON 43) investigating the value of high-dose daunorubicin show OS according to cytogenetic risk in patients >60 years.3 CBF, core-binding factors; CN, normal karyotype (including –Y,–Y); CA rest, other cytogenetic abnormalities; Unfav, MK-, complex cytogenetic abnormalities (at least 3 unrelated cytogenetic abnormalities), monosomies, or partial deletions of chromosome 5 or 7 (del[5q], del[7q], −5, −7), abnormalities of the long arm of chromosome 3 (q21;q26), t(6;9) (p23;q34), t(9;22)(q34;q11.2), or abnormalities involving the long arm of chromosome 11 (11q23); MK, monosomal karyotype; N, numbers; F, failures (death).
Overall survival according to cytogenetic risk in patients above 60 years. Data derived from the HOVON/SAKK study (HOVON 43) investigating the value of high-dose daunorubicin show OS according to cytogenetic risk in patients >60 years.3 CBF, core-binding factors; CN, normal karyotype (including –Y,–Y); CA rest, other cytogenetic abnormalities; Unfav, MK-, complex cytogenetic abnormalities (at least 3 unrelated cytogenetic abnormalities), monosomies, or partial deletions of chromosome 5 or 7 (del[5q], del[7q], −5, −7), abnormalities of the long arm of chromosome 3 (q21;q26), t(6;9) (p23;q34), t(9;22)(q34;q11.2), or abnormalities involving the long arm of chromosome 11 (11q23); MK, monosomal karyotype; N, numbers; F, failures (death).
Patient 3: A 73-year-old man with AML with myelodysplasia-related changes: medical doubts about the feasibility of intensive chemotherapy
A 73-year-old man was admitted to our hospital with blood counts of Hgb 5.9 mmol/L, platelets 53 × 109/L, WBC 3.5 × 109/L, and 12% blasts in the differential. His bone marrow was infiltrated with blasts (27%) that were Sudan Black–positive and showed >50% dysplastic changes in the megakaryocytic and erythroid series. The immunophenotype was consistent with myeloid leukemia. Cytogenetic analysis revealed a trisomy 8, and no molecular abnormalities (NPM1 [nucleophosmin-1] gene mutation, EVI-1 overexpression, FLT3-ITD [internal tandem duplications], CEBPA gene mutations) were detected. Thus, the leukemia was classified as AML with myelodysplasia-related changes. The patient’s performance status was 2, but he had multiple comorbidities such chronic obstructive pulmonary disease, arterial vascular occlusive disease, and diabetes mellitus, and he had also recently been diagnosed with Alzheimer disease. His HCT-IC score was 4, which correlates with an estimated average early death rate on remission-induction chemotherapy of ∼30%. Our team felt that there were too many medical issues and hurdles to confidently recommend an intensive treatment approach.
The patient started treatment with subcutaneous (SC) azacitidine 75 mg/m2 for days 1 to 7 every 28 days in an outpatient setting. Prophylactic antibiotics were administered. The therapy was tolerated well, with only mild gastrointestinal disturbances and local reactions at the injection site noted. After the fourth cycle, the transfusion dependency declined and peripheral blood counts began to recover. Bone marrow examination showed a decrease of blasts to 7% with persistent dysplastic features. Treatment was continued, and after 8 cycles <5% blasts were noted in the marrow smear. He did not show full recovery of his platelet counts to normal values and thus he achieved a CRi. Unfortunately, after cycle 12, the leukemia recurred. At that point, treatment was discontinued and the patient died at 14 months after the initial diagnosis as a result of progressive disease.
Comments about patient 3
In this case, the therapeutic decisions were dictated by performance status and comorbidities. The diagnosis of AML with myelodysplasia-related changes does not a priori classify it as a bad prognostic leukemia. AML with myelodysplasia-related changes is very heterogeneous, thus it lacks independent prognostic significance. The prognosis is determined by underlying cytogenetic and molecular abnormalities.42,43 What are the possibilities in the case a patient is classified as unfit or will not likely benefit from dose-intensive chemotherapy? LDAC (20 mg SC 2× for 10 days for 4-6 weeks) is quite commonly used in these patients in some countries but is less popular in other countries. Treatment with LDAC does not confer considerable toxicity and it produces a higher CR rate than best supportive care (18% vs 1%).44 Although the OS for the LDAC-treated group has been demonstrated to be statistically significantly better, it should be considered that in absolute terms, the therapeutic advantage is marginal and corresponds with a prolongation of OS of only a few months. The benefit is restricted to the minority fraction of patients who achieve a CR (median survival 19 months vs 2 months in nonresponders).44 Furthermore, patients with adverse cytogenetics do not seem to benefit from LDAC. Thus, the OS in patients receiving LDAC is still highly unsatisfactory (median 5 months).
Hypomethylating drugs are considered by many clinicians as an attractive strategy for this patient group. Accumulated experience in myelodysplastic syndromes (MDS) have paved the way for the use of these agents in AML. Two hypomethylating agents—azacitidine and decitabine—have been studied in elderly patients with AML who are not considered candidates for intensive chemotherapy. In a phase 3 trial, azacitidine (75 mg/m2 SC per 7 days for 28 days) was compared with various conventional care regimens (ie, LDAC, intensive chemotherapy, or supportive care) in patients with intermediate-2 and high-risk myelodysplasia. In fact, 113 patients among this series had bone marrow blast percentages of 20% to 29%, and thus the trial included AML with low blast counts only. CR rates were similar for azacitidine when compared with conventional care treatments (18% vs 16%).45 Recently, azacitidine has also been compared with a mix of conventional care regimens (ie, supportive care only, cytoreduction with hydroxyurea, and anthracyclin-Ara-C–based remission-induction chemotherapy) in a study of elderly patients with AML with any blast count. Although azacitidine demonstrated a slight improvement in median OS (10.4 months vs 6.5 months), no statistical significance for the study’s primary end point of OS (P = .08) was achieved. However a preplanned sensitivity analysis censored for subsequent AML treatment showed a benefit in terms of median OS of 12.1 months vs 6.9 months for azacitidine.46
In addition, decitabine 20 mg/m2 daily for 5 days per cycle has been compared with conventional care (either supportive care or LDAC) in a phase 3 trial of 485 patients aged 65 years or older with AML who were considered unfit for intensive chemotherapy. Treatment with decitabine resulted in a higher response rate (CR + CRi 17.8% vs 7.8%) and better survival that reached statistical significance on further follow-up in an unplanned analysis (median OS 7.7 vs 5.0 months).47,48 However, as yet, no trend to a plateau in the survival curves has been apparent in any of these low-intensity regimens. Improvement in performance status and organ function after a successful low-intensity regimen may create a possibility for a curative reduced-intensity allogeneic HSCT. Currently, azacitidine has licensed approval from European Medicines Agency Committee for Medicinal Products for Human Use (EMA) for intermediate-2 and high-risk MDS and AML with 20% to 30% blast cell count, and decitabine has been approved for patients of 65 years and above with AML who are not considered candidates for standard induction therapy. Both azacitidine and decitabine have been approved by the US Food and Drug Administration for all types of MDS including refractory anemia with excess of blasts, and thus also for AML with 20% to 30% of blasts, according the current World Health Organization classification of myeloid neoplasms. Early correlative studies suggest that particular AML genotypes, especially TET2 and DNMT3A-mutated AMLs, may benefit from the use of these epigenetic agents.49,50
A more intensified regimen of decitabine (20 mg/m2 daily for 10 days) was applied to 53 patients (median age 74 years) who were unsuitable for standard chemotherapy, and the outcomes were encouraging.51 The CR rate was 47% and CRi 17%, with 30- and 60-day mortality rates of 2% and 15%, respectively. OS and disease-free survival durations were 55 and 46 weeks (median), respectively. Responses were present in all subgroups regardless of age, cytogenetics, leukocyte count, and antecedent myelodysplasia. The data from this small single-arm study are encouraging, but at this stage they are far from definitive. The sparse comparative data on demethylating agents vs intensive chemotherapy that have been published fail to show a clear advantage for intensive treatment. However, no long-term survival data have been reported after therapy with hypomethylating drugs. A prospective study comparing these agents with intensive treatment is yet to be conducted.
Patient 4: A 72-year-old woman with intermediate-risk AML who declined a recommended intensive chemotherapy approach
At the time of diagnosis, this 72-year-old woman presented with recurrent upper airway infection. She was a widow and had no children and lived a solitary life. Her medical history was unremarkable. A complete blood cell count included a WBC of 3.8 × 109/L, neutrophils 0.4 × 109/L, Hgb 5.7 mmol/L, and platelets 23 × 109/L. Morphologic examination of the bone marrow revealed an AML without maturation, with 85% Sudan Black–positive blasts and immunophenotypic examination consistent with a myeloid leukemia. Cytogenetic evaluation showed a normal 46,XX karyotype. Molecular analysis did not reveal mutations of the NPM1, FLT3, or CEBPA genes, nor EVI-1 overexpression. Thus, the AML was prognostically classified as intermediate risk.
After extensive discussions with this intelligent and fit woman, she declined the proposed option of intensive treatment. She elected to participate in a clinical study that enabled her to be treated in an outpatient setting. She was included in a pharmaceutical-sponsored trial that prospectively compared LDAC alone with LDAC plus an investigational drug. She achieved a CR after 2 treatment cycles that persists for >12 months while she continues to receive treatment. She does not report any significant side effects.
Comments about patient 4
Although this patient would in our view have been suitable for intensive treatment, she deliberately declined this option. Patients should be encouraged to make decisions based on accurate information about the risks and benefits of all available treatment options. Aside from the chances of cure and treatment-related mortality, decisions should also include discussions on living and social circumstances, quality of life issues, and personal expectations in relation to either choice.52 In our patient, individual socioeconomic factors determined her decision and these then had to be taken into account to define the appropriate treatment. Because our patient was interested in receiving a less-intensive treatment, there was an opportunity to enroll her in a clinical trial that, whenever possible, we consider a priority option.
Final considerations
Today, older patients with AML can be offered one of the following treatment options:
Standard induction treatment consisting mostly of a 3+7 regimen of an anthracyclin and Ara-C;
Hypomethylating agents;
Investigational drugs within a clinical trial;
Low-dose Ara-C;
Best supportive care with oral cytostatic drugs like hydroxyurea and/or transfusions.
Obviously, the assessment of the clinical performance and medical condition about whether intensive chemotherapy can be recommended remains a subjective approach that largely depends on the judgment of the medical doctor. Risk-scoring systems as we have discussed could be instrumental in this regard. Geriatric assessments with a focus on cognitive and physical function by either objective measurements or self-reported measures have not been widely studied. However, early studies suggest that they have added value in comparison with performance status and comorbidity evaluations, and thus they may improve the prediction of survival in older patients receiving intensive chemotherapy.19,20
What in general can we learn from the clinical vignettes presented here?
First, we should always, also in patients of older age, make a deliberate therapeutic plan that makes sense to the individual circumstances and try to adhere to the defined plan as much as possible, even though intercurrent problems may urge the modification and temporary deviation from the original intentions.
Second, as an initial priority, it seems useful to follow the same therapeutic principles that we apply in younger adults provided the medical situation of the patient allows for intensive induction chemotherapy. This implies that intensive remission induction chemotherapy is the first choice whenever this is considered realistic and feasible on clinical grounds. Early death is in most studies <15% and does not seem to play a major role in the inferior outcome of elderly patients with AML. In this regard, it is of note that the early death rate in intensively-treated patients has decreased considerably over the last 2 decades, most probably owing to better supportive care.21 A wait-and-see approach with supportive care and cytoreduction with hydroxyurea does not provide a significantly better perspective for the patient with AML in terms of improving quality of life or prolonging survival because none of the basic medical problems will be tackled.28
Third, nonmyeloablative allogeneic HSCT being reasonably well tolerated in terms of early toxicity has shifted the age limit of the applicability of allogeneic HSCT upward. Allogeneic HSCT after reduced-intensity conditioning currently provides antileukemic effectiveness that is not much different from ablative allogeneic HSCT.
Fourth, in a general sense, the molecular features that characterize the risk of AML in middle-aged adults also apply to older adults with AML,37 although the incidence of unfavorable genotypes is significantly more frequent among older adults. These genetic disease-related features of the leukemia furnish clinically informative prognostic insights and thus may offer useful guidance during the therapeutic treatment of an individual patient. For instance, good-risk cytogenetics (core-binding factor leukemias) express a distinctly favorable impact in older patients with AML.3,9 This background information may be reassuring in our treatment approach when intercurrent medical hurdles during the treatment of an older patient are encountered. Conversely, monosomal karyotypes at the unfavorable end of the cytogenetic spectrum carry adverse prognostic value3 (Figure 1).Various studies have established that the favorable effect of the NPM1-mutant genotype in the absence of FLT3 gene mutations for patients treated with intensive chemotherapy protocols also holds up in older patients with AML.3,9,53,54 This genotype exerts a strong positive effect on outcome that is much more apparent than that of individual NPM1 or FLT3 genotypes. Alternately, in some studies, FLT3-ITD, DNMT3A, and ASXL1 gene mutations seem to confer a negative effect on response and survival estimates in older patients with normal cytogenetics.55-57 In this regard, it should be noted that the frequencies of these genotypes are considerably less common in older patients,58 whereas the incidence of particularly unfavorable genotypes (eg, ASXL1, TET2 gene mutations) appear to increase with progressively higher patient age.57,59,59-61 In addition, gene-expression levels have been evaluated for their prognostic value. They use relative cutoff values (high vs low) rather than absolute values and therefore are more difficult to apply as a reference in clinical practice.62 These accumulating genomic data mark the beginning of efforts to better understand and predict responsiveness and refractoriness to antileukemic drugs in individual patients.
Fifth, numerous new drugs are currently emerging from the development pipeline. For example, small drugs targeting a specific oncogenic pathway, a variety of drugs with novel mechanisms of action and/or affecting novel intracellular targets, as well as monoclonal antibodies and antibody conjugates are in clinical development, and some of these may likely enrich the therapeutic arsenal in the near future.
Testing so many emerging new drugs poses a real challenge and urges for new trial designs like the “Pick-a-Winner” concept that should offer the possibility of rationally designed combinations of new drugs.63,64 It would be desirable that major AML trial groups combine efforts in designing rational and complementing trials in close collaboration to accelerate the treatment development of AML that is so urgently needed.
Sixth, we recommend whenever possible to include older AML patients in well-designed clinical trials. This furnishes some guarantee for quality of treatment (eg, protocolized treatment according to state-of-the-art standards), but it also offers the opportunity to contribute to progress in this still devastating disease.65 Especially the elderly unfit and relapsing population of patients with AML are often selected for clinical trials with new therapeutic agents. Many new drugs have failed approval, in part perhaps because the setting in which these drugs are tested has been suboptimal. The study population of relapsed/refractory AML in the older patient population by definition contains some of the most notoriously resistant leukemias. Early clinical trials on new drugs could and should also be actively pursued effectively and informatively in the up-front context in fit, elderly patients with AML and in distinctly genomic-defined AML in which the effect of drugs specifically targeting components of key signal transduction pathways can be investigated.
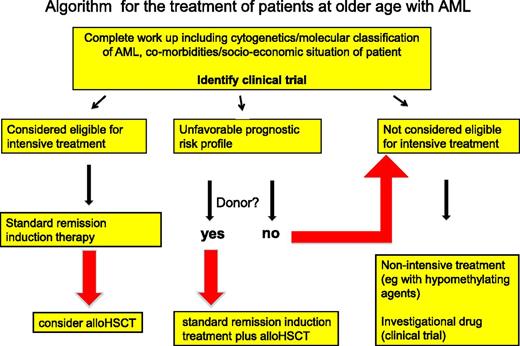
The treatment outcome in older patients is reduced compared with that of younger and middle-aged adults with AML, but this should not be a reason for a fatalistic approach in the older patient. The patient with AML, irrespective of his or her age, deserves the same opportunity for adequate diagnostics—including molecular genetics—that provide a documented substrate for a thoughtfully considered treatment plan (Figure 2).
General algorithm for the treatment of older patients with AML. This algorithm serves as a global guideline and should not be applied dogmatically but with thoughtful consideration of the individual circumstances. Patients eligible for intensive treatment are considered for remission-induction chemotherapy, after which, depending on the response and the risk profile of the leukemia, an allogeneic HSCT as consolidation therapy can be considered. For patients with an unfavorable-risk AML, intensive chemotherapy is mainly considered when a donor for an allogeneic HSCT is available and a subsequent allogeneic HSCT can be foreseen. Otherwise, these unfavorable-risk patients and patients ineligible for intensive chemotherapy will more likely be considered for less-intensive treatment approaches, or for a clinical trial with an interesting investigational agent. A minority of these patients may eventually still proceed to an allogeneic HSCT in case they would show an exceptionally good response to treatment and their general performance status at that point appears to show sufficient improvement so that an allogeneic HSCT is considered feasible. For reasons discussed in the text, we recommend including patients in a clinical trial whenever possible. Medical criteria and dilemmas regarding patient eligibility for intensive chemotherapy are also discussed in the manuscript.
General algorithm for the treatment of older patients with AML. This algorithm serves as a global guideline and should not be applied dogmatically but with thoughtful consideration of the individual circumstances. Patients eligible for intensive treatment are considered for remission-induction chemotherapy, after which, depending on the response and the risk profile of the leukemia, an allogeneic HSCT as consolidation therapy can be considered. For patients with an unfavorable-risk AML, intensive chemotherapy is mainly considered when a donor for an allogeneic HSCT is available and a subsequent allogeneic HSCT can be foreseen. Otherwise, these unfavorable-risk patients and patients ineligible for intensive chemotherapy will more likely be considered for less-intensive treatment approaches, or for a clinical trial with an interesting investigational agent. A minority of these patients may eventually still proceed to an allogeneic HSCT in case they would show an exceptionally good response to treatment and their general performance status at that point appears to show sufficient improvement so that an allogeneic HSCT is considered feasible. For reasons discussed in the text, we recommend including patients in a clinical trial whenever possible. Medical criteria and dilemmas regarding patient eligibility for intensive chemotherapy are also discussed in the manuscript.
Authorship
Contribution: G.O. and B.L. both wrote the article.
Conflict-of-interest disclosure: G.O. has a consultancy with J&J, Novartis, Celgene, and ARIAD, and has received research funding from Novartis and Celgene. The remaining author declares no competing financial interests.
Correspondence: Gert Ossenkoppele, VU University Medical Center, Department of Hematology, De Boelelaan 1117, PO Box 7057, Amsterdam, 1007 MB, The Netherlands; e-mail: g.ossenkoppele@vumc.nl.

![Figure 1. Overall survival according to cytogenetic risk in patients above 60 years. Data derived from the HOVON/SAKK study (HOVON 43) investigating the value of high-dose daunorubicin show OS according to cytogenetic risk in patients >60 years.3 CBF, core-binding factors; CN, normal karyotype (including –Y,–Y); CA rest, other cytogenetic abnormalities; Unfav, MK-, complex cytogenetic abnormalities (at least 3 unrelated cytogenetic abnormalities), monosomies, or partial deletions of chromosome 5 or 7 (del[5q], del[7q], −5, −7), abnormalities of the long arm of chromosome 3 (q21;q26), t(6;9) (p23;q34), t(9;22)(q34;q11.2), or abnormalities involving the long arm of chromosome 11 (11q23); MK, monosomal karyotype; N, numbers; F, failures (death).](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/125/5/10.1182_blood-2014-08-551499/4/m_767f1.jpeg?Expires=1763475338&Signature=rhZtG68N1aBgr0ERAapmwDINdGFkJDY0NRJXU0PGOxnBxZy~4hZ76XtNAEfndDionORktvTPhDyhK59S8a~0mhanACoB5UGLWUM1RvXrKF0FyDbh7-dLtQPSxb1RWN~5SH9lGByb23ZnXpj7LVezy19ednqfCmhXQPmlbH6WZJJ5G1~2NveoDxzmvvUKHFDcHsXbR3pV8jW2Ty2tj~xCK5XeMmdJf68YvEeI68HDfWQm~VsRbGfQ2o62voBams1cqRXjct-nqMcPcgWfLi3--kV-0uAPOJ2xiZ8gn5jm3YrrVRg1L0JLmVzmqUUYfaU408WhdFIeG0WuIh2UoYKDqQ__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)