Key Points
We elucidate a molecular mechanism by which thyroid hormones sustain TCL survival.
We demonstrate that the membrane receptor of THs, integrin αvβ3, constitutes a potential target for TCL.
Abstract
The interaction of lymphoid tumor cells with components of the extracellular matrix via integrin αvβ3 allows tumor survival and growth. This integrin was demonstrated to be the membrane receptor for thyroid hormones (THs) in several tissues. We found that THs, acting as soluble integrin αvβ3 ligands, activated growth-related signaling pathways in T-cell lymphomas (TCLs). Specifically, TH-activated αvβ3 integrin signaling promoted TCL proliferation and angiogenesis, in part, via the upregulation of vascular endothelial growth factor (VEGF). Consequently, genetic or pharmacologic inhibition of integrin αvβ3 decreased VEGF production and induced TCL cell death in vitro and in human xenograft models. In sum, we show that integrin αvβ3 transduces prosurvival signals into TCL nuclei, suggesting a novel mechanism for the endocrine modulation of TCL pathophysiology. Targeting this mechanism could constitute an effective and potentially low-toxicity chemotherapy-free treatment of TCL patients.
Introduction
T-cell lymphomas (TCLs) are a molecular and clinical heterogeneous group of lymphoproliferative disorders broadly classified according to their origin into T-cell lymphoblastic lymphoma (from precursor T cells) and peripheral TCLs (PTCL) (from mature T cells).1 Among the later, the most common varieties are the nodal types, including PTCL not otherwise specified (PTCL-NOS), anaplastic large-cell lymphoma (ALCL), and angioimmunoblastic TCL (AITL). Although some subtypes may follow a more benign prolonged course, the vast majority of PTCL patients have poor prognoses due to the combination of an aggressive clinical course and the lack of specific treatments originally designed for B-cell lymphomas.2-4
The cellular microenvironment plays an essential role in the pathogenesis and progression of lymphomas.5,6 The interaction of lymphoma cells with ligand molecules in the extracellular matrix (ECM) is responsible for the colonization of specific anatomical sites as well as for lymphoma-cell survival and aggressiveness.6 The principal ECM receptors are integrins that mediate cell attachment to other cells and the ECM. Engagement of integrins by ECM ligands induces a variety of intracellular signals that regulate migration, differentiation, proliferation, and apoptosis. Altered integrin expression or signaling is frequently associated with lymphoma-cell invasion and growth.7
Integrins are α/β heterodimers with different ligand specificity. Integrin αvβ3, expressed in malignant cells, osteoclasts, and dividing endothelial and vascular smooth muscle cells,8 binds ECM components (eg, vitronectin) and is also the membrane receptor for thyroid hormones (THs).9-11 Therefore, THs exert their biological actions through simultaneous binding of nuclear receptors and integrin αvβ3. TH-induced activation of integrin αvβ3 triggers a signaling cascade that modulates the transcription of a set of genes that will complement the nuclear-initiated program.10-12 We recently demonstrated that physiological levels of THs can stimulate the proliferation of murine TCLs through complementary intracellular pathways involving nuclear and membrane receptors, ultimately leading to protein kinase C, extracellular signal-regulated kinase 1/2 (ERK1/2), and nuclear factor κB (NF-κB) activation.13,14 In this context, a hypothyroid state would be beneficial for TCL patients; however, this strategy is associated with potentially harmful effects that preclude its clinical application. We hypothesized that targeting integrin αvβ3 could help to overcome this limitation; however, the mechanistic contributions of integrin αvβ3 to human TCL survival are unknown.
Herein, we characterize the role of TH-induced integrin αvβ3 activation in the survival of malignant T cells. We determine the signaling and transcriptional program triggered by TH binding to integrin αvβ3 on TCLs leading to increase proliferation and angiogenesis. We also demonstrate that targeting integrin αvβ3 is sufficient to induce lymphoma regression in vivo.
Methods
TCL cell lines and primary samples
Jurkat and CUTLL1 immature TCL/leukemia cells were obtained from Dr Roxana Schillaci (Instituto de Biología y Medicina Experimental, CONICET, Argentina) and Dr Adolfo Ferrando (Columbia University, New York, NY), respectively; MJ, HuT78, and Mac2A were obtained from the American Type Culture Collection; Karpas299 and SUDHL1 were obtained from the DMSZ; and OCI-Ly12 and OCI-Ly13.2 were obtained from the Ontario Cancer Institute, Canada. Cells were cultured in RPMI 1640 medium, supplemented with 10% fetal bovine serum, 2 mM glutamine, and antibiotics (from Invitrogen). We conducted monthly tests for Mycoplasma sp. and other contaminants and quarterly cell identification by single-nucleotide polymorphism. Primary samples were obtained from the Weill Cornell Medical College Biobank under institutional review board approval 0107004999A006.
Tissue culture treatments
Cell lines were treated at different times with free or agarose-bound hormones at physiological concentration (T3 or T3-AG = 1 nM; T4 or T4-AG = 100 nM; Sigma-Aldrich). For [3H]-TdR incorporation and migration assays, cells were synchronized by 12-hour serum starvation (in RPMI) as published previously.13 For all other experimental procedures, cells were cultured 24 hours before treatments in RPMI with 10% TH–depleted serum (Sunny-Laboratory).
Human TCL xenograft models
Experiments involving animals were approved by the Institutional Animal Care and Use Committee of Weill Cornell Medical College. For human cell line xenografts, female and male severe combined immunodeficiency (SCID) (NOD.CB17-Prkdcscid/J) mice 6 to 10 weeks old were obtained from Taconic Farms. A cohort of mice was subcutaneously injected in the left flank with 2 × 107 CUTLL1 cells previously transfected with small interfering RNA (siRNA) against ITGAV, ITGB3, or noncoding sequence. Another cohort of mice was subcutaneously injected in the left flank with 107 OCI-Ly12 cells. Tumor volume was measured every other day for the duration of the experiment, and the area under the tumor growth curve (AUC) was calculated. When OCI-Ly12 xenografts reached a palpable size (∼75 to 100 mm3), mice were randomized into 2 treatment arms to receive vehicle (35% PEG300, 5% Tween-80, and 65% dextrose 5% in water) or cilengitide (125 mg/kg per day, MedKoo) by intraperitoneal injection. For patient-derived xenografts, previously established patient-derived tumorgrafts (1 mm3 blocks) were subcutaneously expanded into NSG (NOD.Cg-Prkdcscid Il2rgtm1Wjl/SzJ) mice 6 to 8 weeks old obtained from our own colony. Tumor assessment and treatments were done as before. Mice from both models were weighed every other day and killed by cervical dislocation under anesthesia when at least 20% of tumors reached 20 mm in any dimension. At the end of the experiment, tumor and other tissues were harvested, weighed, and macroscopically examined for signs of tissue damage.
Statistical analysis
Means of the different experimental groups were analyzed for statistical significance with the software GraphPad PRISM 4.0 (GraphPad Software), using unpaired 2-tailed Student t test or 2-way analysis of variance followed by Tukey’s analysis. Differences between means were considered significant if P < .05. Results are expressed as mean ± standard error of the mean (SEM). Correlation results from patient samples were analyzed using Pearson test for sampled Gaussian populations (2 tailed) and Spearman test for nonparametric correlation (VEGFA vs ITGAV in ALCL patients samples).
Additional methods are described in supplemental Materials and methods (available on the Blood Web site).
Results
TCLs express canonical and noncanonical TH receptors
We demonstrated that THs induce murine TCL proliferation in vitro13,14 by activating both nuclear and membrane receptors. To determine whether THs also contribute to maintaining proliferation in human malignant T cells, we first characterized the expression of nuclear TRα and TRβ, and the putative membrane integrin αvβ3 receptors, in a panel of TCL cell lines. This panel included immature TCL/leukemia (Jurkat and CUTLL1) and mature TCLs comprising the cutaneous TCL cell lines MJ and HuT78, the PTCL-NOS cell line OCI-Ly12, the ALCL anaplastic lymphoma kinase (ALK)-negative cell lines OCI-Ly13.2 and Mac2a, and the ALCL ALK-positive cell lines Karpas299 and SUDHL1. All these cells expressed THRA, ITGAV, and ITGB3 (Figure 1A), but not THRB, at similar or higher levels than normal peripheral and tonsillar T cells. TH receptors were also expressed at the protein level as shown for representative cell lines (Figure 1B). This suggests that integrins and nuclear TH receptors are functional to normal and malignant T cells.
THs mediate proliferative effects in TCL cell lines. (A) mRNA levels of ITGAV, ITGB3, and THRA in a panel of TCL cell lines in comparison with mRNA levels in normal T cells. Results shown are the mean ± SEM of n = 3 independent experiments. (B) Protein levels of integrin αvβ3 and TRαβ obtained by flow cytometry in CUTLL1, SUDHL1, OCI-Ly12, and HuT 78 cell lines. Results are representative of n = 3 experiments. (C) CUTLL1 cells treated for 24 hours with free or agarose-bound T3 (1 nM) and T4 (100 nM) or a combination of the same concentrations of both free (TH) or AG-coupled (TH-AG) hormones were evaluated by Cell Titer Blue assay. Results shown represent the percent of proliferative cells respective to CT. (D) Cell proliferation after 24-hour treatment with TH or TH-AG compared with CT (dashed line) in the complete TCL cell line panel. (E) Evaluation of DNA synthesis by [3H]TdR incorporation of CUTLL1, HuT 78, and OCI-Ly12 cells after 24 hours of hormone treatment vs CT. (F) Cyclin mRNA expression levels in CUTLL1 cells by quantitative reverse-transcription polymerase chain reaction (qRT-PCR) after 2-, 6-, 12-, and 18-hour treatment with TH-AG (right) and free TH (left) compared with CT. (G) Cyclin D1 mRNA expression levels in HuT 78 and OCI-Ly12 cells by qRT-PCR after a 2-, 6-, and 12-hour treatment with TH-AG and free TH compared with CT. (H) PCNA protein levels by flow cytometry in CUTLL1 cells (right) and mRNA (left) levels in CUTLL1, HuT 78, and OCI-Ly12 cells after 24-hour treatment with free TH and AG-bound TH compared with CT. qRT-PCR results shown are the mean ± SEM of at least n = 3 independent experiments. For PCNA protein levels, a representative of 4 experiments is displayed. CT, control.
THs mediate proliferative effects in TCL cell lines. (A) mRNA levels of ITGAV, ITGB3, and THRA in a panel of TCL cell lines in comparison with mRNA levels in normal T cells. Results shown are the mean ± SEM of n = 3 independent experiments. (B) Protein levels of integrin αvβ3 and TRαβ obtained by flow cytometry in CUTLL1, SUDHL1, OCI-Ly12, and HuT 78 cell lines. Results are representative of n = 3 experiments. (C) CUTLL1 cells treated for 24 hours with free or agarose-bound T3 (1 nM) and T4 (100 nM) or a combination of the same concentrations of both free (TH) or AG-coupled (TH-AG) hormones were evaluated by Cell Titer Blue assay. Results shown represent the percent of proliferative cells respective to CT. (D) Cell proliferation after 24-hour treatment with TH or TH-AG compared with CT (dashed line) in the complete TCL cell line panel. (E) Evaluation of DNA synthesis by [3H]TdR incorporation of CUTLL1, HuT 78, and OCI-Ly12 cells after 24 hours of hormone treatment vs CT. (F) Cyclin mRNA expression levels in CUTLL1 cells by quantitative reverse-transcription polymerase chain reaction (qRT-PCR) after 2-, 6-, 12-, and 18-hour treatment with TH-AG (right) and free TH (left) compared with CT. (G) Cyclin D1 mRNA expression levels in HuT 78 and OCI-Ly12 cells by qRT-PCR after a 2-, 6-, and 12-hour treatment with TH-AG and free TH compared with CT. (H) PCNA protein levels by flow cytometry in CUTLL1 cells (right) and mRNA (left) levels in CUTLL1, HuT 78, and OCI-Ly12 cells after 24-hour treatment with free TH and AG-bound TH compared with CT. qRT-PCR results shown are the mean ± SEM of at least n = 3 independent experiments. For PCNA protein levels, a representative of 4 experiments is displayed. CT, control.
Activation of nuclear and membrane TH receptors induces proliferation of TCLs
We determined the functionality of TH receptors by exposing CUTLL1 cells to physiological concentrations of free and agarose-coupled (cell-impermeable) T3 and T4. Agarose-bound THs bind exclusively to the membrane receptor without binding to nuclear receptors,9 providing a discriminatory tool to activate exclusively integrin αvβ3. We found that free and TH-AG hormones significantly increased the proliferation of CUTLL1 cells (Figure 1C). A higher effect was obtained by the combination at physiological dose and ratio of both T3 and T4 or T3-AG and T4-AG compared with each hormone alone (Figure 1C). A similar proliferative effect was found across immature and mature TCL cell lines that showed an increase in proliferative activity ranging from 30% to 50% (Figure 1D).
To further characterize this effect, we measured DNA synthesis and expression of molecular markers of cell-cycle progression in representative immature (CUTLL1) and mature (HuT78 and OCI-Ly12) cells. We found that TH and TH-AG significantly increased DNA synthesis (Figure 1E). This was associated with a cell-cycle progression–dependent induction of cyclins upon hormone addition to arrested cells. An initial increase of d-type cyclins, required for the progression from G0 to G1, was followed by subsequent increase in cyclin E2 (G1 to S and S phase) and cyclin B1 (G2 to M) in CUTLL1 cells exposed to either TH or TH-AG (Figure 1F). A similar pattern for cyclins of the D and E families was found in Hut78 and OCI-Ly12 cells (Figure 1G and supplemental Figure 1A). These effects were also linked to hormone-mediated upregulation of proliferating cell nuclear antigen (PCNA) levels, a central component in DNA synthesis and replication (Figure 1H and supplemental Figure 1B). No induction of apoptosis was found when TCL cells were cultured in the presence or absence of THs (supplemental Figure 1C). Taken together, these data suggest that THs acting through membrane and nuclear receptors constitute proliferative signals for TCLs.
TH-activated integrin αvβ3 dimer sustains TCL proliferation
Physiological levels of THs increase the proliferation of TCLs through membrane and nuclear receptors. However, targeting integrin αvβ3, instead of TH production or the ubiquitous THRA, would constitute a more feasible anti-lymphoma strategy to translate to patients. The TH binding site on integrin αvβ3 is in close proximity to the Arg-Gly-Asp (RGD) recognition site9,11 ; accordingly, a competing RGD peptide completely abrogated the effect of TH-AG on cell proliferation and decreased a significant fraction of the prosurvival effect of free TH (Figure 2A). In the absence of integrin αvβ3 ligand, the RGD peptide alone had no effect on cell proliferation (Figure 2A). To further validate integrin αvβ3 targeting as a therapeutic strategy in TCL, we transfected CUTLL1 cells with siRNA to integrin αv (si-ITGAV) and/or integrin β3 (si-ITGB3) and evaluated THs effect on these conditions vs nontargeting sequence siRNA (si-CT) as control. After 24 and 48 hours, si-ITGAV and si-ITGB3 effectively and selectively (vs other RGD integrins expressed in TCLs) knocked down integrin αvβ3 (Figure 2B and supplemental Figure 2A-B). Both si-ITGAV and si-ITGB3 decreased CUTLL1 cell growth in presence of TH (Figure 2C), suggesting that the heterodimer is necessary for the proliferative effect of TH on TCLs. DNA synthesis analysis demonstrated that, although si-ITGAV, si-ITGB3, and si-ITGAV+si-ITGB3 did not significantly affect CUTLL1 proliferation in the absence of αvβ3 ligand, TH-induced proliferation was completely abrogated (Figure 2D). No differences were observed when both si-ITGAV and si-ITGB3 were used in combination, indicating that downregulation of any of the components impaired the activity of the integrin heterodimer (Figure 2D). The effect of si-ITGAV and si-ITGB3 (vs si-CT) in the mature TCL HuT78 and OCI-Ly12 cells was similar to that with CUTLL1 cells (Figure 2E-F and supplemental Figure 2B), suggesting that the αvβ3 dimer is critical for TH-induced proliferation in most T-cell malignances.
Integrin αvβ3 is the membrane receptor for THs in human TCL. (A) Blockade of the TH-mediated proliferative effect by 1 nM RGD peptide, added 10 minutes before 24-hour hormone treatment, was analyzed by Cell Titer Blue assay. (B) CUTLL1 cells were transfected by electroporation with siRNA against ITGAV (si-ITGAV), ITGB3 (si-ITGB3), or noncoding siRNA as control (si-CT). mRNA levels of target genes were analyzed by qRT-PCR 24 and 48 hours posttransfection. (C) Effect of ITGAV and ITGB3 knockdown on CUTLL1 cell growth by Trypan Blue staining. (D-F) Knockdown of ITGAV and/or ITGB3 abrogates the TH proliferative effect as measured by [3H]TdR incorporation (D) and cell proliferation by Cell Titer Blue assay (E) after 24 hours of TH treatment in CUTLL1 cells. Similar results were found in mature TCL, HuT 78, and OCI.Ly12 cells (F). (G) Effect of 250 ng/mL vitronectin ligand and/ or THs on cell proliferation in CUTLL1, HuT 78, and OCI-Ly12 cells measured by [3H]TdR incorporation after 24-hour treatment. (H) Growth of OCI-Ly12 cells cultured in an artificial ECM system that offers an RGD ligand, in the presence or absence of physiological levels of TH and in the presence or absence of the inhibitor of integrin αvβ3 cilengitide. Cell proliferation and clustering was measured by microscopical examination. Representative photographs (scale bar, 100 μm) of 4 replicate experiments are shown.
Integrin αvβ3 is the membrane receptor for THs in human TCL. (A) Blockade of the TH-mediated proliferative effect by 1 nM RGD peptide, added 10 minutes before 24-hour hormone treatment, was analyzed by Cell Titer Blue assay. (B) CUTLL1 cells were transfected by electroporation with siRNA against ITGAV (si-ITGAV), ITGB3 (si-ITGB3), or noncoding siRNA as control (si-CT). mRNA levels of target genes were analyzed by qRT-PCR 24 and 48 hours posttransfection. (C) Effect of ITGAV and ITGB3 knockdown on CUTLL1 cell growth by Trypan Blue staining. (D-F) Knockdown of ITGAV and/or ITGB3 abrogates the TH proliferative effect as measured by [3H]TdR incorporation (D) and cell proliferation by Cell Titer Blue assay (E) after 24 hours of TH treatment in CUTLL1 cells. Similar results were found in mature TCL, HuT 78, and OCI.Ly12 cells (F). (G) Effect of 250 ng/mL vitronectin ligand and/ or THs on cell proliferation in CUTLL1, HuT 78, and OCI-Ly12 cells measured by [3H]TdR incorporation after 24-hour treatment. (H) Growth of OCI-Ly12 cells cultured in an artificial ECM system that offers an RGD ligand, in the presence or absence of physiological levels of TH and in the presence or absence of the inhibitor of integrin αvβ3 cilengitide. Cell proliferation and clustering was measured by microscopical examination. Representative photographs (scale bar, 100 μm) of 4 replicate experiments are shown.
TH activates the integrin αvβ3 dimer in the presence of ECM ligands
To evaluate the magnitude of TH activity on integrin αvβ3–mediated proliferation in the presence of its ECM ligand, we cultured TCL cells in the presence of vitronectin. Vitronectin alone had a tendency to increase lymphoma cell proliferation, but to a lesser extent than that observed with THs (Figure 2G). When added together, vitronectin did not compete out the TH effect, and for HuT78 cells, the combination of vitronectin and TH provided a greater proliferative effect than each ligand alone (Figure 2G).
To deeper determine whether the presence of cellular and noncellular stromal components influences the activation of integrin αvβ3 by TH, as well as the antiproliferative effect of integrin αvβ3 targeting, we developed a tridimensional culture system that mimics cellular and noncellular components of ECM in mature TCLs. We adapted a hydrogel system15,16 by combining functionalized polyethylene glycol with a crosslinker peptide containing an integrin αvβ3 binding site and a collagenase degradable sequence. As inert stromal cellular component, we used irradiated human tonsil–derived HK follicular dendritic cells (supplemental Figure 3). OCI-Ly12 cells were grown in this culture system in presence and absence of physiological levels of TH and in presence and absence of the specific RGD peptidomimetic integrin αvβ3 inhibitor cilengitide.17 We measured cell proliferation and clustering by microscopic examination. In these artificial ECM conditions, OCI-Ly12 proliferated between 2 and 3 times more than in regular tissue culture conditions (data not shown). Moreover, in the presence of physiological levels of TH, clustering and proliferation increased (Figure 2H), suggesting that both RGD stromal components and TH cooperate toward TCL proliferation in conditions that resemble the natural microenvironment of these tumors. Conversely, cilengitide abrogated the clustering and proliferative effect of the artificial ECM and the THs (Figure 2H).
TH-activated integrin αvβ3 regulates prosurvival transcriptional programs
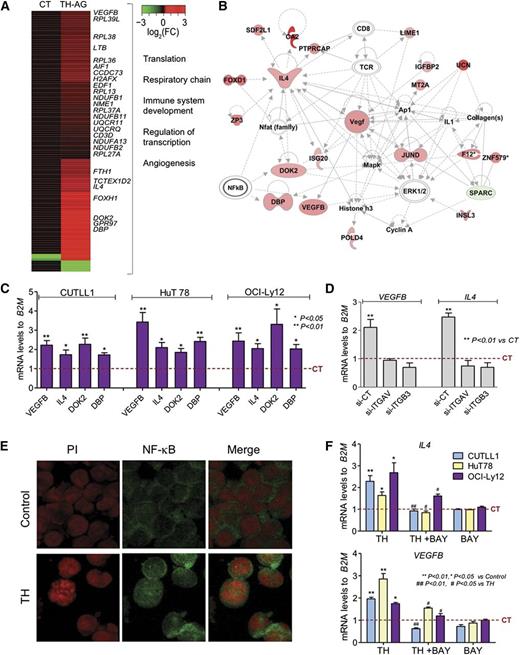
To determine the mechanism by which TH-activated αvβ3 integrins maintain the proliferation of TCLs, we analyzed transcriptional changes induced by TH-AG (vs agarose alone as CT) in CUTLL1 cells by RNA sequencing. We identified 118 upregulated and 5 downregulated transcripts (Figure 3A and supplemental Table 1) involved in “translation” (eg, RPL13, RPL27A, RPL36), “respiratory chain” (eg, NUDFB1/2, NDUFA13, UQCR11), “angiogenesis” (eg, VEGFB), “lymphocyte proliferation/differentiation,” and “DNA replication/transcription” (eg, DBP, IL4, EDF1, DOK2) (Figure 3A-B). Previously, we identified ERK1/2 and NF-κB as effectors of TH-activated αvβ3 integrins in murine TCLs.14 Pathway analysis of these TH-AG–mobilized genes also suggested an association with ERK1/2 and NF-κB (Figure 3B). We therefore validated the upregulation of genes presenting consensus NF-κB binding sites in their promoter region (ie, DBP, VEGFB, IL4, and DOK2) in independent biological experiments. We found that TH-activated αvβ3 integrin signaling upregulated these genes in CUTLL1 cells and in the mature HuT78 and OCI-Ly12 cell lines (Figure 3C). To determine whether free THs could bypass the integrin receptor in upregulating target genes, we analyzed the upregulation of IL-4 and VEGFB by free THs in the presence and absence of integrin αvβ3. We found that CUTLL1 transfected with either si-ITGAV or si-ITGB3 (vs si-CT) failed to upregulate IL-4 and VEGFB expression upon TH stimulation (Figure 3D). These data indicate that TH-dependent regulation of these genes is due to the activation of αvβ3 and further inhibition of this integrin abrogates this signaling event.
THs acting at the membrane receptor initiate a transcriptional program. (A) A graphical heatmap representation of changes (log2 fold change) in gene expression of CUTLL1 cells after treatment with TH-AG vs CT (AG alone); n = 3 independent samples for each condition. Selected Gene Ontology categories for upregulated genes are shown on the right. (B) The top network identified by Ingenuity Pathway Analysis on mobilized genes by AG-bound hormones treated cells vs CT. (C) Validation of a select number of genes from the network shown in panel B, 24 hours posttreatment with TH-AG in immature CUTLL1 cells and mature TCL cell lines, HuT 78, and OCI-Ly12. (D) Regulation of IL-4 and VEGFB mRNA levels after 2 hours of treatment with free TH in the presence and absence of integrin αvβ3. (E) NF-κB localization by confocal microscopy of CUTLL1 cells treated or not (Control) with THs for 15 minutes. Representative photographs (scale bar, 20 μm). (F) Effect of 1 µM of NFκB inhibitor, BAY 11-7082, on TH-mediated regulation of IL-4 and VEGFB genes in CUTLL1, HuT 78, and OCI-Ly12 cells.
THs acting at the membrane receptor initiate a transcriptional program. (A) A graphical heatmap representation of changes (log2 fold change) in gene expression of CUTLL1 cells after treatment with TH-AG vs CT (AG alone); n = 3 independent samples for each condition. Selected Gene Ontology categories for upregulated genes are shown on the right. (B) The top network identified by Ingenuity Pathway Analysis on mobilized genes by AG-bound hormones treated cells vs CT. (C) Validation of a select number of genes from the network shown in panel B, 24 hours posttreatment with TH-AG in immature CUTLL1 cells and mature TCL cell lines, HuT 78, and OCI-Ly12. (D) Regulation of IL-4 and VEGFB mRNA levels after 2 hours of treatment with free TH in the presence and absence of integrin αvβ3. (E) NF-κB localization by confocal microscopy of CUTLL1 cells treated or not (Control) with THs for 15 minutes. Representative photographs (scale bar, 20 μm). (F) Effect of 1 µM of NFκB inhibitor, BAY 11-7082, on TH-mediated regulation of IL-4 and VEGFB genes in CUTLL1, HuT 78, and OCI-Ly12 cells.
Pathway analysis and our previous findings in murine TCL models14 suggest that NF-κB mediates downstream of the TH-activated αvβ3 integrin signaling. We now confirm that in human TCLs, TH also induced the activation and therefore nuclear translocation of NF-κB (Figure 3E). Moreover, administration of the specific NF-κB inhibitor BAY-11-7082 significantly abrogated the upregulation of VEGFB and IL4 on both immature (CUTLL1) and mature (HuT78 and OCI-Ly12) TCL cell lines (Figure 3F). BAY-11-7082 alone was unable to decrease VEGFB or IL4 (Figure 3E). This suggests that NF-κB is downstream of TH-activated αvβ3 integrin signaling and regulates the transcription of genes involved in proliferation and angiogenesis.
TH-activated integrin αvβ3 induces VEGF expression and activity
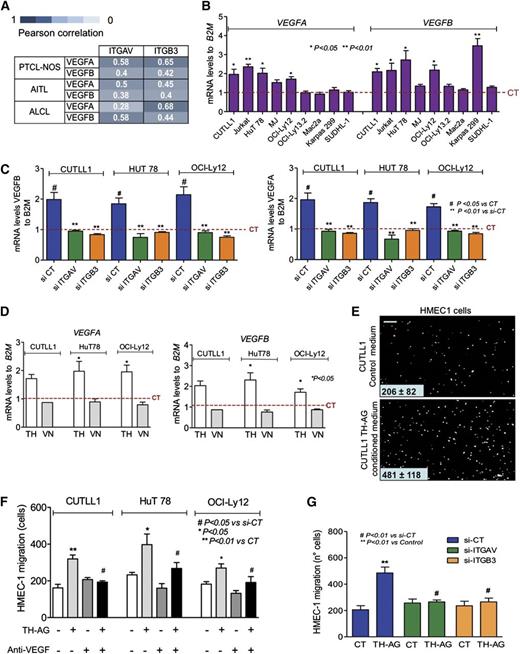
VEGF expression and angiogenesis correlate with survival and prognosis in PTCL-NOS and AITL patients.18-20 VEGFB is a member of a larger family of growth factors that also includes VEGFA, VEGFC, VEGFD, and PLGF.21 In addition to VEGFB, the only member expressed in samples of a series of TCL patients (74 PTCL-NOS, 30 ALCL-ALK positive, 24 ALCL-ALK negative, and 41 AITL) was VEGFA (supplemental Figure 4A). Therefore, we analyzed whether VEGFA, like VEGFB, was under the control of integrin αvβ3 in TCL. We transfected CUTTL1 cells with either si-ITGAV or si-ITGB3 (vs si-CT) followed by TH stimulation. Similar to IL-4 and VEGFB, VEGFA was upregulated upon TH stimulation, exclusively in cells with intact integrin αvβ3 dimer (supplemental Figure 4B), indicating that this member is also part of the integrin αvβ3–dependent VEGF family in TCLs. Moreover, NF-κB inhibition using BAY-11-7082 abrogated this effect (supplemental Figure 4C). To determine whether this association was present in our series of TCL patients, we analyzed the expression of THRA, ITGAV, and ITGB3 (supplemental Figure 5A-C) and the correlation between VEGFA and VEGFB vs ITGAV and ITGB3 (supplemental Figure 5D). We observed a statistically significant positive correlation between ITGAV and ITGB3 with VEGFB and VEGFA in almost all of the TCL subtypes (Figure 4A and supplemental Figure 5D), suggesting a functional association in primary lesions.
THs contribute to TCL malignant phenotype through angiogenesis induction. (A) Pearson correlation between ITGAV and ITGB3 with VEGFB and VEGFA levels in TCL patients. (B) mRNA levels of VEGFA and VEGFB in the panel of immature and mature TCLs after 24-hour treatment with TH-AG compared with CT. (C) Transcript abundance of VEGFA (right) and VEGFB (left) was evaluated in CUTLL1, HuT78, and OCI-Ly12 cell lines transfected for 48 hours with siRNA against ITGAV (si-ITGAV), ITGB3 (si-ITGB3), or noncoding sequence (si-CT) and then treated for 24 hours with TH-AG or agarose alone (as CT). (D) Transcript abundance of VEGFB (right) and VEGFA (left) was evaluated in CUTLL1, HuT78, and OCI-Ly12 cell lines after 24-hour treatment with 250 ng/mL vitronectin (VN). (E) Induction of HMEC1 cell migration by conditioned medium from CUTLL1 cells treated or not with TH-AG. Representative photographs (scale bar, 60 μm) of the endothelial cells that migrate through the chamber membrane in the presence of CT or conditioned medium. (F) Quantitation of HMEC1 cell migration by conditioned medium from CUTLL, HuT 78, and OCI-Ly12 cells treated or not with TH-AG and preincubated with the anti-VEGF bevacizumab (10 μg/mL) vs vehicle. (G) Cell migration quantitation of HMEC1 cells in the presence or absence of conditioned medium from si-RNA–transfected CUTLL1 cells treated with TH-AG vs CT for 24 hours. Mean ± SEM of at least 3 independent experiments are shown.
THs contribute to TCL malignant phenotype through angiogenesis induction. (A) Pearson correlation between ITGAV and ITGB3 with VEGFB and VEGFA levels in TCL patients. (B) mRNA levels of VEGFA and VEGFB in the panel of immature and mature TCLs after 24-hour treatment with TH-AG compared with CT. (C) Transcript abundance of VEGFA (right) and VEGFB (left) was evaluated in CUTLL1, HuT78, and OCI-Ly12 cell lines transfected for 48 hours with siRNA against ITGAV (si-ITGAV), ITGB3 (si-ITGB3), or noncoding sequence (si-CT) and then treated for 24 hours with TH-AG or agarose alone (as CT). (D) Transcript abundance of VEGFB (right) and VEGFA (left) was evaluated in CUTLL1, HuT78, and OCI-Ly12 cell lines after 24-hour treatment with 250 ng/mL vitronectin (VN). (E) Induction of HMEC1 cell migration by conditioned medium from CUTLL1 cells treated or not with TH-AG. Representative photographs (scale bar, 60 μm) of the endothelial cells that migrate through the chamber membrane in the presence of CT or conditioned medium. (F) Quantitation of HMEC1 cell migration by conditioned medium from CUTLL, HuT 78, and OCI-Ly12 cells treated or not with TH-AG and preincubated with the anti-VEGF bevacizumab (10 μg/mL) vs vehicle. (G) Cell migration quantitation of HMEC1 cells in the presence or absence of conditioned medium from si-RNA–transfected CUTLL1 cells treated with TH-AG vs CT for 24 hours. Mean ± SEM of at least 3 independent experiments are shown.
VEGFA and VEGFB may contribute to TCL proliferation by 2 mechanisms: one involving the expression of VEGF receptors in TCL cells (autocrine),22,23 and another due to sustained angiogenesis through a paracrine effect on the tumor microenvironment.21 Therefore, a TH-dependent increase of VEGFA and/or VEGFB may promote TCL growth by inducing proliferation of the malignant population as well as by promoting angiogenesis. To determine the extent of this effect, we first analyzed whether TH-AG could increase the expression of VEGFB and VEGFA in our panel of malignant T cells. Similarly to CUTLL1, TH-AG increased VEGFB messenger RNA (mRNA) levels in Jurkat, HuT-78, OCI-Ly12, and Karpas299 and increased VEGFA in Jurkat, HuT78, MJ, and OCI-Ly12 cells (Figure 4B). This effect was dependent on integrin αvβ3, because siRNA to ITGAV or ITGB3 completely abrogated TH-AG–induced upregulation of VEGFB and VEGFA expression in CUTLL1, HuT-78, and OCI-Ly12 cells (Figure 4C), suggesting that in most TCLs, the expression of VEGF ligands depends on the TH-αvβ3 integrin axis activity. Moreover, these effects were not induced by the ECM integrin αvβ3 ligand vitronectin (Figure 4D).
To further characterize the paracrine consequences of TH-induced integrin-dependent VEGF upregulation, we analyzed the effect of a conditioned medium from CUTLL1, HuT78, or OCI-Ly12 cells treated with TH-AG on the migration of endothelial cells, a VEGF-dependent effect. Conditioned medium was placed in the lower compartment of a dual-chamber device separated from the upper compartment containing HMEC1 endothelial cells by a polycarbonate porous membrane. We then quantified the HMEC1 endothelial cells that migrated to the lower compartment and found a significant increase in the number of endothelial cells that migrated to the lower compartment when TH-AG–treated TCL conditioned media was used (Figure 4E-F). To confirm that this effect was VEGF dependent, we conducted this experiment adding the anti-VEGF antibody bevacizumab to HMEC1 culture medium. We found that anti-VEGF effectively impaired the HMEC1 migration effect of the conditioned media (Figure 4F). Bevacizumab had a negligible effect on the migration of endothelial cells culture with control media (Figure 4F). This suggests that VEGF upregulation leads to a fully functional ligand. Furthermore, this effect was completely abrogated when the conditioned medium was obtained from CUTLL1 cells transfected with siRNA to ITGAV or ITGB3 and treated with TH-AG for 24 hours (Figure 4G and supplemental Figure 6).
VEGF mediates TH-induced TCL proliferation
To analyze the possible role of TH-induced VEGF in an autocrine circuit, we first determined the effect of recombinant VEGF on the proliferation of TCL cells. We found that recombinant VEGF induced a significant increase in the proliferation of CUTLL1, HuT78, and OCI-Ly12 cells (Figure 5A). We then evaluated the effect of the anti-VEGF antibody, bevacizumab, and of a specific inhibitor of VEGFR, axitinib, on the proliferative action of TH on these cell lines. Both bevacizumab and axitinib abrogated the proliferation induced by THs in both immature and mature TCL cells (Figure 5B), thus indicating that VEGF and VEGFR contribute to the TH-induced proliferation of TCL cells.
Autocrine effect of TH-induced VEGF in TCL proliferation. (A) TCL proliferation after 24-hour treatment with recombinant VEGF vs CT. (B) DNA synthesis measured by [3H]TdR incorporation in CUTLL1, HuT 78, and OCI-Ly12 cells exposed to TH and treated with the anti-VEGF bevacizumab or vehicle and the VEGFR inhibitor axitinib or vehicle. Results shown are the mean ± SEM of independent triplicates. (C) CUTLL1 cells were transfected with si-ITGAV, si-ITGB3, or si-CT and injected into SCID mice (n = 4 for each treatment). Tumor growth from day 4 to 15 after implantation was measured by the AUC. (D) Levels of VEGFA and active caspase-3 by immunohistochemistry staining in tissue sections of the tumors obtained from panel A. Representative photographs are shown (scale bar, 50 μm). (E) Representative photographs of CD31 staining of tissue sections from the CUTLL1 tumors. Quantification of the blood vessel area (lumen) from the CUTLL1 tumors (right).
Autocrine effect of TH-induced VEGF in TCL proliferation. (A) TCL proliferation after 24-hour treatment with recombinant VEGF vs CT. (B) DNA synthesis measured by [3H]TdR incorporation in CUTLL1, HuT 78, and OCI-Ly12 cells exposed to TH and treated with the anti-VEGF bevacizumab or vehicle and the VEGFR inhibitor axitinib or vehicle. Results shown are the mean ± SEM of independent triplicates. (C) CUTLL1 cells were transfected with si-ITGAV, si-ITGB3, or si-CT and injected into SCID mice (n = 4 for each treatment). Tumor growth from day 4 to 15 after implantation was measured by the AUC. (D) Levels of VEGFA and active caspase-3 by immunohistochemistry staining in tissue sections of the tumors obtained from panel A. Representative photographs are shown (scale bar, 50 μm). (E) Representative photographs of CD31 staining of tissue sections from the CUTLL1 tumors. Quantification of the blood vessel area (lumen) from the CUTLL1 tumors (right).
We characterized the functional consequences of integrin αvβ3 inhibition in vivo in a TCL xenograft model of CUTLL1 cells transfected with si-CT, si-ITGAV, or si-ITGB3. CUTLL1 transfected with either si-ITGAV or si-ITGB3 developed smaller tumors than si-CT–transfected cells (Figure 5C). TCL xenografts from si-ITGAV– or si-ITGB3–transfected cells showed a marked decrease in VEGFA and increase in activated caspase-3 (Figure 5D and supplemental Figure 7). Additionally, CD31 immunostaining of the tumor vasculature revealed an anatomically defective vascularization in these lymphomas (Figure 5E). TCLs from si-ITGAV and si-ITGB3 CUTLL1 cells showed blood vessels with a smaller lumen area (si-ITGAV = 0.40 ± 0.12 × 10−3; si-ITGB3 = 0.34 ± 0.08 × 10−3 vs si-CT = 1.07 ± 0.25 × 10−3 mm2, P < .01), suggesting a diminished angiogenic potential in tumors from cells with either integrin αV or β3 knockdown. Taken together, these results indicate that THs induce integrin αvβ3–dependent production of fully functional VEGFs with autocrine and paracrine effects.
Inhibition of integrin αvβ3 decreases the proliferation of TCL in vivo
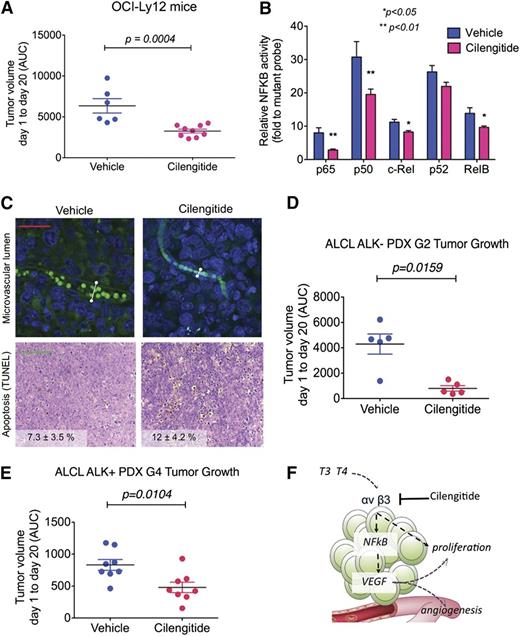
To determine whether this pathway can be therapeutically capitalized upon for the treatment of TCL patients, we assessed the effect of cilengitide, a selective αvβ3 integrin inhibitor currently in clinical use.17 We developed a PTCL-NOS model by xenografting OCI-Ly12 cells into SCID mice. Once OCI-Ly12 tumors reached a palpable size, we treated them with cilengitide at a dose equivalent to half of the maximum tolerated dose in human patients or vehicle. At the end of the treatment, cilengitide-treated mice showed significantly smaller tumors compared with vehicle-treated CTs (P < .001; Figure 6A). This effect was associated with decreased activation of the NF-κB pathway (Figure 6B), decreased microvascular lumen size (0.44 ± 0.22 × 10−3 vs 0.97 ± 0.55 × 10−3 mm2, vehicle vs cilengitide, respectively, P < .05; Figure 6C, upper) and increased apoptosis (7.3 ± 3.5% vs 12.0 ± 4.2%, vehicle vs cilengitide, respectively, P < .05; Figure 6C lower). There was no toxicity associated with this treatment as determined by body weight and macroscopic examination of mouse tissue.
Pharmacologic inhibition of integrin shows anti-lymphoma effect in PTCL-NOS and ALCL mouse models. (A) Tumor volume (measured by AUC, from day 1 to day 20) in OCI-Ly12 xenografted mice treated with vehicle vs cilengitide. (B) DNA-binding capacity of NF-κB family members assessed by an enzyme-linked immunosorbent assay–based assay in extracted nuclear fractions of disaggregated lymphoma tissues from the tumors obtained in panel A. (C) Microvascular lumen (by intravascular isolectin) and apoptotic bodies (by terminal deoxynucleotidyltransferase-mediated dUTP nick end labeling assay) in representative tissue sections of the tumors from vehicle- and cilengitide-treated OCI-Ly12 mice. Scale bar, 25 μm (upper) and 100 μm (lower). The white lines show representative maximum lumen size measurements. (D-E) AUC of tumor growth of ALCL ALK-negative (D) and ALCL ALK-positive (E) patient-derived lymphomas in NSG mice treated with vehicle vs cilengitide. (F) Cartoon representation of TH regulation of TCL prosurvival pathways via the activation of αvβ3 integrins.
Pharmacologic inhibition of integrin shows anti-lymphoma effect in PTCL-NOS and ALCL mouse models. (A) Tumor volume (measured by AUC, from day 1 to day 20) in OCI-Ly12 xenografted mice treated with vehicle vs cilengitide. (B) DNA-binding capacity of NF-κB family members assessed by an enzyme-linked immunosorbent assay–based assay in extracted nuclear fractions of disaggregated lymphoma tissues from the tumors obtained in panel A. (C) Microvascular lumen (by intravascular isolectin) and apoptotic bodies (by terminal deoxynucleotidyltransferase-mediated dUTP nick end labeling assay) in representative tissue sections of the tumors from vehicle- and cilengitide-treated OCI-Ly12 mice. Scale bar, 25 μm (upper) and 100 μm (lower). The white lines show representative maximum lumen size measurements. (D-E) AUC of tumor growth of ALCL ALK-negative (D) and ALCL ALK-positive (E) patient-derived lymphomas in NSG mice treated with vehicle vs cilengitide. (F) Cartoon representation of TH regulation of TCL prosurvival pathways via the activation of αvβ3 integrins.
To determine the effect of cilengitide in TCLs that more closely resemble the patients’ lymphoma heterogeneity and are less manipulated than cells kept in culture, we developed 2 models of patient-derived xenografts. We xenografted ALCL ALK-negative passage 2 lymphomas and ALCL ALK-positive passage 4 lymphomas into NSG mice. Once these tumors reached a palpable size, we treated them with cilengitide or vehicle as before. At the end of the treatment, cilengitide-treated mice showed significantly smaller tumors compared with vehicle-treated CTs (P = .0159 and P = .0104 for ALCL ALK negative and ALCL ALK positive, respectively; Figure 6D-E). There was no toxicity associated with this treatment as determined by body weight and macroscopic examination of mouse tissue.
Discussion
Here, we show that physiological concentrations of THs acting through integrin αvβ3 induce angiogenesis and proliferation of immature and mature TCL cells. We characterized the transcriptional program triggered upon activation of the integrin αvβ3 by TH that is ultimately linked to the induction of angiogenic and cell proliferation genes such as VEGF, an NF-κB–dependent mechanism (Figure 6F). Moreover, we found that pharmacologic inhibition of integrin αvβ3 reduces the proliferation of TCLs in preclinical models (Figure 6F), including patient-derived xenografts of ALCL tumors.
Integrin αvβ3 is the receptor for ECM proteins including vitronectin, Cyr61, osteopontin, fibronectin, and fibrinogen as well as for soluble factors like THs. TH also binds 1 or more isoforms of a nuclear receptor, TR. Both TH receptors, TRα and the integrin αvβ3, were expressed in TCL cells and patient samples, indicating that they are potentially sensitive to the circulating levels of T3 and T4 normally present in the tumor microenvironment. Moreover, recent data suggested that TCL cells could have an enriched TH microenvironment by a mechanism that involves epigenetic transcriptional control of DIO3 (type III deiodinase). This enzyme plays an essential role in regulating TH inactivation in tissues, and it was found hypermethylated and repressed in malignant T cells.24 Transcript analysis of our TCL panel showed undetectable levels of DIO3 and DIO2 and low expression of DIO1 (not shown). Nonetheless, using physiological levels of T3 and T4, we demonstrated that THs are growth factors for TCL. These effects were mediated by free and cell-impermeable hormones and included increments in cell proliferation, metabolism, and cell-cycle induction and progression.
In the characterization of the programs induced by TH on integrin αvβ3, we found genes related to signaling cascades for proliferation and angiogenesis, such as IL-4, DOK2, and VEGFB, that present NF-κB binding sites in their promoter regions. We further confirmed the participation of NF-κB in the signaling cascade triggered upon integrin αvβ3 activation. IL-4 is a proliferative cytokine for PTCL25 and T-cell acute lymphoblastic leukemia cells, in the later through a mammalian target of rapamycin–dependent regulation of cell-cycle progression.26 The adaptor DOK2, frequently overexpressed in ALCL,27 acts as a common substrate for multiple tyrosine kinases and induces cell migration, in part, by interacting with integrin β3.28 Possibly the most therapeutically actionable finding is the αvβ3-dependent upregulation of VEGFB and VEGFA. Malignant T cells produce and express VEGF receptors, thus supporting autocrine and paracrine VEGF-mediated pathways in lymphangiogenesis.22,23 VEGF and VEGF receptors are expressed in vascular and lymphoma cells of AILT and PTCL-NOS patients.20,22,23 Moreover, the expression of VEGF was associated with tumor staging, bone marrow invasion, and prognosis in PTCL-NOS patients.19 We found that TH-dependent αvβ3-activation increased VEGFB and VEGFA expression and secretion, which in turn activated endothelial cell migration as well as immature and mature TCL proliferation, establishing an autocrine effect. Conversely, anti-VEGF antibody treatment impaired TH-induced endothelial cell migration and lymphoma proliferation.
The proliferative action of THs was abrogated by knockdown of αV and/or β3 integrins and by an integrin αvβ3–selective RGD peptidomimetic compound, thus indicating that the heterodimeric integrin αvβ3 is sufficient to transduce the proliferative effects of THs. Engagement of integrin αvβ3 by the ECM ligand vitronectin did not decrease the TH effect of proliferation. Moreover, in organoid models of TCL, simultaneous activation of integrin αvβ3 by RGD-containing ligands and TH resulted in increased proliferation and clustering. Subsequent exposure of these organoids to the integrin αvβ3 inhibitor cilengitide29,30 decreased the proliferation and clustering effect. Furthermore, cilengitide showed anti-lymphoma effect in cell-line–derived and patient-derived xenograft models of mature TCLs. Targeting the integrin αvβ3 in TCL and patient-derived xenografts, by either a genomic or a pharmacologic approach, significantly reduced tumor growth, an effect partially mediated by defective angiogenesis. This could represent a potential strategy to test in the clinical setting, because cilengitide is currently being tested in phase 2/3 clinical trials for solid tumors.30 In this regard, our finding that integrin αvβ3 mediates proliferation and survival of TCL would make it an attractive target for therapy, alone or in combination with conventional schedules.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
The authors thank Dr Vanina Medina and Mr Jayeshkumar Patel for help with imaging and Alejandra Paulazo for technical assistance.
L.C. is the Raymond and Beverly Sackler Scholar and Scholar of the American Society of Hematology. This work was supported by the National Institutes of Health, National Cancer Institute (R21 CA185236) (L.C. and A.S.), the National Research Council of Argentina (PIP-CONICET 00275) (G.A.C.), the University of Buenos Aires (UBACYT 20020130100289BA) (G.A.C.), the National Agency for Science and Technology (ANPCYT, PICT 20081858) (G.A.C.) (PICT-Raíces 2012-1328) (G.A.C. and L.C.), the Malvin Peace Sevin Research Scholar Award (L.C.), the Irma T. Hirschl Career Scientist Award (L.C.), the Cutaneous Lymphoma Foundation CLARIONS Research Award (L.C.), the Italian Association for Cancer Research Special Program in Clinical Molecular Oncology (5x1000 no. 10007) (G.I.), and the Jorge Oster Award from the Bunge and Born Foundation (F.C.).
Authorship
Contribution: F.C., L.C., and G.A.C. designed research; F.C., M.C.D.F., S.N.Y., H.A.S., M.A., M.B., F.T., H.A., and Y.F.T. performed experiments; B.I., R.N.F., and G.I. contributed vital reagents and acquired and managed patient samples; F.C., T.F., A.S., L.C., and G.A.C. analyzed and interpreted data and performed statistical analysis; F.C., L.C., and G.A.C. wrote the manuscript; and L.C. and G.A.C. supervised the study.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Leandro Cerchietti, Weill Cornell College of Medicine, Cornell University, 1300 York Ave, New York, NY 10065; e-mail: lec2010@med.cornell.edu; and Graciela Alicia Cremaschi, Neuroimmunomodulation and Molecular Oncology Department, BIOMED-UCA-CONICET, Avda Alicia Moreau de Justo 1600, 3rd Floor, Buenos Aires, Argentina; e-mail: gacremaschi@gmail.com.
References
Author notes
L.C. and G.A.C. contributed equally to this study.

![Figure 1. THs mediate proliferative effects in TCL cell lines. (A) mRNA levels of ITGAV, ITGB3, and THRA in a panel of TCL cell lines in comparison with mRNA levels in normal T cells. Results shown are the mean ± SEM of n = 3 independent experiments. (B) Protein levels of integrin αvβ3 and TRαβ obtained by flow cytometry in CUTLL1, SUDHL1, OCI-Ly12, and HuT 78 cell lines. Results are representative of n = 3 experiments. (C) CUTLL1 cells treated for 24 hours with free or agarose-bound T3 (1 nM) and T4 (100 nM) or a combination of the same concentrations of both free (TH) or AG-coupled (TH-AG) hormones were evaluated by Cell Titer Blue assay. Results shown represent the percent of proliferative cells respective to CT. (D) Cell proliferation after 24-hour treatment with TH or TH-AG compared with CT (dashed line) in the complete TCL cell line panel. (E) Evaluation of DNA synthesis by [3H]TdR incorporation of CUTLL1, HuT 78, and OCI-Ly12 cells after 24 hours of hormone treatment vs CT. (F) Cyclin mRNA expression levels in CUTLL1 cells by quantitative reverse-transcription polymerase chain reaction (qRT-PCR) after 2-, 6-, 12-, and 18-hour treatment with TH-AG (right) and free TH (left) compared with CT. (G) Cyclin D1 mRNA expression levels in HuT 78 and OCI-Ly12 cells by qRT-PCR after a 2-, 6-, and 12-hour treatment with TH-AG and free TH compared with CT. (H) PCNA protein levels by flow cytometry in CUTLL1 cells (right) and mRNA (left) levels in CUTLL1, HuT 78, and OCI-Ly12 cells after 24-hour treatment with free TH and AG-bound TH compared with CT. qRT-PCR results shown are the mean ± SEM of at least n = 3 independent experiments. For PCNA protein levels, a representative of 4 experiments is displayed. CT, control.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/125/5/10.1182_blood-2014-07-587337/4/m_841f1.jpeg?Expires=1769804036&Signature=OvQxr7tL3BtQMQBbm8B7ZhyiIWAGJGUHCktIUtk3wyyryfGbL2-TdvOv2lCRVDSIpSYXXqeB4vDzF8KUqI9u8qYo8egjdXWlOAH5nmNPkryE5r9ESxWd2VqI3bB747lobYeOjbW15ZdY5hjwREfyIvpXImCJeCM1Ek~OnsXQlnSeCrmGTup4gNbsr5kkc3jPX5Kv03xduB0BJTjVIZ4mjDt5czDjOgsFAD-gMHXSW-X74mq5C0hqVCMe80C5n8y0TrNr6CbzCP80TETIQvwigwbg55uQAz2cwMexCuzvAnzUrH9V5q3rV9ehiiu6afksuiB~Qbv3yPb4q5PUTX9nYQ__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
![Figure 2. Integrin αvβ3 is the membrane receptor for THs in human TCL. (A) Blockade of the TH-mediated proliferative effect by 1 nM RGD peptide, added 10 minutes before 24-hour hormone treatment, was analyzed by Cell Titer Blue assay. (B) CUTLL1 cells were transfected by electroporation with siRNA against ITGAV (si-ITGAV), ITGB3 (si-ITGB3), or noncoding siRNA as control (si-CT). mRNA levels of target genes were analyzed by qRT-PCR 24 and 48 hours posttransfection. (C) Effect of ITGAV and ITGB3 knockdown on CUTLL1 cell growth by Trypan Blue staining. (D-F) Knockdown of ITGAV and/or ITGB3 abrogates the TH proliferative effect as measured by [3H]TdR incorporation (D) and cell proliferation by Cell Titer Blue assay (E) after 24 hours of TH treatment in CUTLL1 cells. Similar results were found in mature TCL, HuT 78, and OCI.Ly12 cells (F). (G) Effect of 250 ng/mL vitronectin ligand and/ or THs on cell proliferation in CUTLL1, HuT 78, and OCI-Ly12 cells measured by [3H]TdR incorporation after 24-hour treatment. (H) Growth of OCI-Ly12 cells cultured in an artificial ECM system that offers an RGD ligand, in the presence or absence of physiological levels of TH and in the presence or absence of the inhibitor of integrin αvβ3 cilengitide. Cell proliferation and clustering was measured by microscopical examination. Representative photographs (scale bar, 100 μm) of 4 replicate experiments are shown.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/125/5/10.1182_blood-2014-07-587337/4/m_841f2.jpeg?Expires=1769804036&Signature=LEZuwrDmmP73~WVnOKvaXflfI6Jf70NMtukqffm1GI3sFbQW2o-FUzIUWABF63h3ibBgi3BTAWFSqsosFp3w0s1tp6PIedKuPEB6jnGoWFLdYeV~6CgDIQZSq1M1plaDMN~n-QZrW7HTLX6oPFFuTs0kNyUCDfNiAo-iopEEnACA-zwV4qIuF5zk1eHILtVGqmM0bFF-y~7SEO8bR2Vq5hpGksNdD8SNC4fKXk7gs9b4mjbi4vTjw41SIacIQ9pf-GqgO-jfWEZMy5iezO85Dds~MP1-9WyhfdqRf0Uf67NGWtiNsQVhx7fhzzaFadngF3jBlaL1YMHVga86JtA8OQ__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
![Figure 5. Autocrine effect of TH-induced VEGF in TCL proliferation. (A) TCL proliferation after 24-hour treatment with recombinant VEGF vs CT. (B) DNA synthesis measured by [3H]TdR incorporation in CUTLL1, HuT 78, and OCI-Ly12 cells exposed to TH and treated with the anti-VEGF bevacizumab or vehicle and the VEGFR inhibitor axitinib or vehicle. Results shown are the mean ± SEM of independent triplicates. (C) CUTLL1 cells were transfected with si-ITGAV, si-ITGB3, or si-CT and injected into SCID mice (n = 4 for each treatment). Tumor growth from day 4 to 15 after implantation was measured by the AUC. (D) Levels of VEGFA and active caspase-3 by immunohistochemistry staining in tissue sections of the tumors obtained from panel A. Representative photographs are shown (scale bar, 50 μm). (E) Representative photographs of CD31 staining of tissue sections from the CUTLL1 tumors. Quantification of the blood vessel area (lumen) from the CUTLL1 tumors (right).](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/125/5/10.1182_blood-2014-07-587337/4/m_841f5.jpeg?Expires=1769804036&Signature=jzPvBzRdYLz-LMd43ybSymeD09O50pRqaZdZJYU23ZJ7ZsfDpqXVfFvLKiICPzZMHva-xTAYcYm1Sz~Y6p6QeliQ~Xy7JhRooosSmReScCyzifZJKaqFL2WJd-hdFM8t-IWGOH1mr9KANfvJ3ZnRUe6IFrJGej7gbUbS0shtU3ghHOZ10ZCg-oez8nkrJCtgYRtcpuIJypkP-k~ZIhSXiTdd2VGIsFqXLyo4iKzJouWSO2bH3NzYuwXKtxWOe0~VH2Trg3aKUlSzJt93LRA20PUQmhAi-0El3j5Uttybx8WJYJOoG3LAVLeK~0qqLtfnrgWmWgQjw2b6WyIwFEnTdg__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
This feature is available to Subscribers Only
Sign In or Create an Account Close Modal