In this issue of Blood, Lecchi et al report that lifelong abnormal bleeding episodes in 2 brothers are the result of a homozygous mutation in the gene encoding the P2Y12 receptor that disrupts adenosine diphosphate (ADP)–promoted platelet aggregation.1
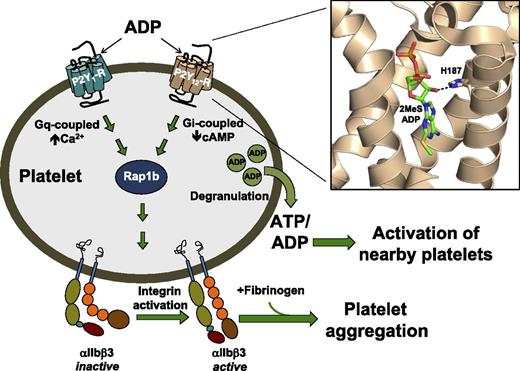
ADP simultaneously binds to 2 P2Y receptors, P2Y1 and P2Y12, leading to sustained activation of Rap1b and a conformation change of αIIbβ3 integrins from an inactive to an active form. Activated αIIbβ3 binds fibrinogen to form a platelet aggregate. Activation of the platelet also promotes degranulation, thereby releasing ATP and ADP to activate nearby platelets and amplify aggregation. (Inset) His187 in the P2Y12 receptor is hydrogen bonded to the 2′-OH of 2MeSADP, which ultimately leads to activation of P2Y12 receptor–mediated signaling pathways. The 2 brothers with a severe bleeding history detailed by Lecchi et al have a homologous His187Gln mutation that disrupts receptor activation. cAMP, cyclic adenosine 5′-monophosphate.
ADP simultaneously binds to 2 P2Y receptors, P2Y1 and P2Y12, leading to sustained activation of Rap1b and a conformation change of αIIbβ3 integrins from an inactive to an active form. Activated αIIbβ3 binds fibrinogen to form a platelet aggregate. Activation of the platelet also promotes degranulation, thereby releasing ATP and ADP to activate nearby platelets and amplify aggregation. (Inset) His187 in the P2Y12 receptor is hydrogen bonded to the 2′-OH of 2MeSADP, which ultimately leads to activation of P2Y12 receptor–mediated signaling pathways. The 2 brothers with a severe bleeding history detailed by Lecchi et al have a homologous His187Gln mutation that disrupts receptor activation. cAMP, cyclic adenosine 5′-monophosphate.
The platelet activity of ADP arises from concomitant stimulation of 2 different P2Y receptor subtypes: the Gq-coupled P2Y1 receptor and the Gi-coupled P2Y12 receptor (see figure). The P2Y1 receptor induces platelet shape change and initiates platelet activation, whereas the P2Y12 receptor amplifies and sustains activation; importantly, the activation of either of these pathways alone is insufficient to cause rapid aggregation. The P2Y12 receptor is the major Gi-coupled receptor in platelets and functions as a predominant gatekeeper in platelet physiology by stabilizing platelet aggregation caused by many initiators, including ADP (through the P2Y1 receptor), serotonin, and thromboxane A2. Not surprisingly, this receptor is the primary target of current antiplatelet therapy. The Gq and Gi signaling pathways converge to promote activation of the small G protein, Rap1b, ultimately leading to a conformational change in αIIbβ3 from an inactive to an active form. The binding of fibrinogen to activated αIIbβ3 cross-links platelets. Vesicle degranulation consequently releases proaggregatory molecules (including adenosine triphosphate [ATP] and ADP), resulting in a positive feedback loop in which nearby platelets are recruited to the prethrombus.
The work of Lecchi et al studying platelets from 2 brothers containing a mutation (His187Gln) in the P2Y12 receptor provides new understanding of how this cornerstone signaling protein functions at the molecular level. The P2Y1 receptor in these platelets functions normally because ADP-promoted shape change occurs. In contrast, the authors discovered that, whereas the P2Y12 receptor is expressed at the platelet surface at normal levels and binds ADP with only modestly reduced affinity, it fails to activate even at high concentrations of ADP. Thus, neither P2Y12 receptor–dependent inhibition of cyclic adenosine 5′-monophosphate (AMP) accumulation nor promotion of ATP release from dense granules occurs, and the platelets exhibit markedly lower and reversible aggregation in response to up to 20 μM ADP. Thus, the studies of Lecchi et al reveal a homozygous mutation in the P2Y12 receptor that results in severe crippling of its capacity to undergo activation.
The recently published structures of the P2Y12 receptor in complex with the agonist 2MeSADP and the antagonist AZD1283 provide exciting new snapshots of the atomic architecture of this platelet signaling protein and reveal unique characteristics of this 7-transmembrane–spanning receptor compared with other receptor structures.2,3 For example, the fifth transmembrane spanning domain (TM5) of this G protein–coupled receptor (GPCR) is distinctively straight and slightly tilted relative to the plasma membrane. When the receptor:agonist complex is viewed laterally, 2MeSADP is oriented nearly vertically, with the adenine ring of 2MeSADP located deep within the binding pocket and the phosphate groups near the top of the pocket where they interact with positive residues in the amino terminus and extracellular loops. His187, which is the focus of the work of Lecchi et al, is located in TM5, where it forms a hydrogen bond with the 2′-OH of the ribose ring of 2MeSADP but does not interact with AZD1283, a reversible-binding antagonist.
Although full understanding of the structural mechanisms underlying receptor activation requires more investigation, the phenotype of the mutant suggests that His187 plays an important role in the ADP-promoted conformational switch to the activated state. The structures of the agonist- and antagonist-bound P2Y12 receptor are extremely useful, but because the agonist-bound structure was generated in the absence of bound G protein or G protein mimic,2 it may not fully reflect the activated state. Structural studies with the β2-adrenergic receptor show that the G protein impacts the conformation of the intracellular region of the receptor.4 That said, substantial insights can be gleaned from these P2Y12 receptor structures, and the biochemical studies of Lecchi et al take advantage of this knowledge to provide molecular insight at the atomic level in a clinically relevant problem.
Other function-modifying mutations in the P2Y12 receptor have been reported in patients with bleeding diathesis: Arg256Gln and Arg265Trp (separate mutations in the 2 alleles of the P2Y12 gene from a single patient), Pro258Thr, Lys174Glu, Arg122Cys, and Pro341Ala.5-9 The phenotypes of these mutations fall into 3 groups: (1) Arg256Gln, Arg265Trp, and Arg122Cys have little to no effect on 2MesADP binding, but block receptor activation; (2) Lys174Glu markedly reduces both 2MeSADP-binding affinity and receptor function; and (3) Pro341Ala is located in the postsynaptic density 95/disc large/zonula occludens-1 (PDZ)–binding motif at the carboxyl terminus and alters receptor trafficking. In the absence of structural information, mechanistic interpretations of the effects of these mutations on the receptor were unclear, but these results can now be reexamined in light of the P2Y12 receptor structure. Arg256 hydrogen bonds with the α phosphate of 2MeSADP (this is surprising because the mutation does not appear to alter the affinity of the nucleotide), neither Lys174 nor Pro258 contacts the ligand but both are likely involved in conformational switching, and Arg122 is located in the highly conserved D(E)RY motif that is important in receptor activation. Lastly, Arg256 is pointing out to solvent in the agonist structure, but moves toward TM5 in the antagonist structure and forms a hydrogen bond with AZD1283, suggesting it also is involved in conformational switching.
Two decades have passed since the physiological action of ADP on platelets was shown to occur through simultaneous activation of 2 different GPCR subtypes that signal via 2 distinct heterotrimeric G protein–mediated signaling pathways. The recent x-ray crystal structures of the predominant of these ADP-activated receptors, the P2Y12 receptor, have provided snapshots of this key mediator of platelet aggregation at the atomic level. The work of Lecchi et al reveals a homozygous mutation in this receptor that results in a bleeding disorder, and their biochemical studies provide an excellent example of how structural biology can inform both mechanistic and clinical investigation. In the process, their work provides insight into how an agonist-bound receptor translates binding energy into conformational switching to the activated state.
Conflict-of-interest disclosure: The author declares no competing financial interests.