Key Points
Two patients with bleeding diathesis had dysfunctional platelet P2Y12R for ADP, attributable to homozygous His187Gln mutation.
These studies delineate a region of transmembrane 5 of P2Y12R that is required for normal function after ADP binding.
Abstract
Defects of the platelet P2Y12 receptor (P2Y12R) for adenosine diphosphate (ADP) are associated with increased bleeding risk. The study of molecular abnormalities associated with inherited qualitative defects of the P2Y12R protein is useful to unravel structure-function relationships of the receptor. We describe the case of 2 brothers, sons of first cousins, with lifelong history of abnormal bleeding, associated with dysfunctional P2Y12R and a previously undescribed missense mutation in the encoding gene. ADP (4-20 µM)–induced aggregation of patients’ platelets was markedly reduced and rapidly reversible. Other agonists induced borderline-normal aggregation. Inhibition of vasodilator-stimulated phosphoprotein phosphorylation and prostaglandin E1–induced increase in cyclic adenosine monophosphate (cAMP) by ADP was impaired, whereas inhibition of cAMP increase by epinephrine was normal. [3H]PSB-0413, a selective P2Y12R antagonist, bound to a normal number of binding sites; however, its affinity, and that of the agonists ADP and 2-methylthio-adenosine-5′-diphosphate, was reduced. Patients’ DNA showed a homozygous c.847T>A substitution that changed the codon for His-187 to Gln (p.His187Gln). Crystallographic data and molecular modeling studies indicated that His187 in transmembrane 5 is important for agonist and nucleotide antagonist binding and located in a region undergoing conformational changes. These studies delineate a region of P2Y12R required for normal function after ADP binding.
Introduction
P2Y12, which maps to chromosome 3q21-q25, is 1 of the 2 receptors for adenosine diphosphate (ADP) that are expressed by platelets.1,2 It is a G inhibitory protein (Gi)-coupled receptor that plays an important role both in hemostasis and thrombosis. This role is demonstrated by the observations that patients with inherited defects of the receptor display a bleeding diathesis2,3 and patients with clinical manifestations of atherothrombosis are protected from major adverse cardiovascular events by P2Y12 receptor (P2Y12R) inhibitors.4
Congenital P2Y12R defects are autosomal recessive disorders, associated with qualitative or quantitative abnormalities of the receptor.2 Affected patients display a bleeding diathesis of variable severity, characterized by mucocutaneous bleeding and excessive postsurgical or posttraumatic blood loss. The most typical platelet function abnormality of the disorder, which should raise the diagnostic suspicion of the disease, is the failure by ADP, even at very high concentrations (>10 μM), to induce full and irreversible platelet aggregation.2,5 Tests that evaluate the degree of inhibition of adenylyl cyclase by ADP, by measuring either the platelet levels of cyclic adenosine monophosphate (cAMP) or the phosphorylation of vasodilator-stimulated phosphoprotein (VASP) after the exposure of platelets to prostaglandin (PG) E1 or other stimuli for adenylyl cyclase, should be used to confirm the diagnosis.2,6
The study of molecular abnormalities associated with inherited qualitative defects of the P2Y12R protein is useful to unravel structure-function relationships of the receptor. Mutations in the region that spans the transition between transmembrane helix (TM) 6 and extracellular loop (EL) 3 are associated with receptor dysfunction despite normal ligand binding, suggesting that this region of the molecule plays a role in signal transduction.2,7-9 A heterozygous mutation, predicting a lysine to glutamate (p.Lys174Glu) substitution in the P2Y12R, was identified in 1 patient with reduced and reversible aggregation in response to ADP and an ∼50% reduction in binding of agonist radioligand [3H]2-methylthio-adenosine-5′-diphosphate (2MeSADP).10 Considering that Lys174 is situated in the EL2 of the P2Y12R, adjacent to Cys175, which may be important for the integrity of the ADP binding site on the receptor,11 and that a hemagglutinin-tagged p.Lys174Glu P2Y12R variant showed surface expression in Chinese hamster ovary cells, it was hypothesized that the p.Lys174Glu mutation inhibits ADP binding to the receptor. Recently reported crystallographic data12,13 do not show a direct involvement of Lys174 in agonist binding but rather a role in stabilizing the receptor conformation through interresidue ionic interactions and/or in affecting the approach of the ligand to its binding site. In one patient, with no personal history of abnormal bleeding, reduced expression of P2Y12R was associated with the heterozygous mutation p.Pro341Ala, in a putative postsynaptic density 95/disc large/zonula occludens-1–binding motif, most likely as a consequence of significantly compromised P2Y12R recycling.14
Here, we report the case of an index patient and his brother, with an inherited dysfunctional P2Y12R associated with a normal number of platelet binding sites but reduced affinity for the P2Y12R antagonist radioligand [3H]2-Propylthioadenosine-5′-adenylic acid (1,1-chloro-1-phosphonomethyl-1-phosphonyl)anhydride ([3H]PSB-0413),15,16 ADP, and 2MeSADP, and with homozygous c.847T>A substitution, which changed the codon for His187 to Gln (p.His187Gln).
Methods
Patients and controls
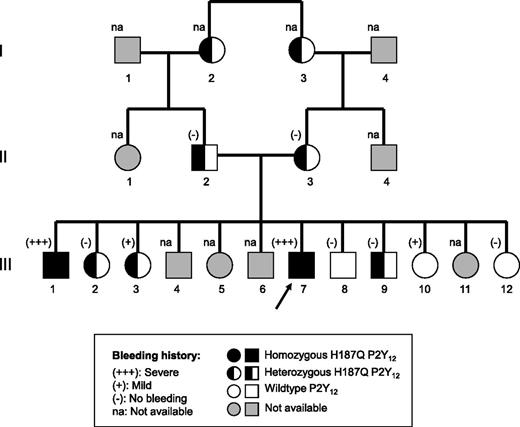
The proband (III-7 in Figure 1) is a 45-year-old man living in Germany, who belongs to a large family of Turkish origin (Figure 1). He presented with recurrent epistaxis, postoperative bleeding after tooth extraction, thyroid explantation because of thyroid hyperplasia, and spleen explantation, consequent to spleen rupture in a car accident. He had normal platelet count (307 × 109/L), screening coagulation tests (prothrombin time and activated partial thromboplastin time), and plasma levels of von Willebrand factor antigen and ristocetin cofactor activity. Serum thromboxane B2 levels and the platelet contents of serotonin, ADP, adenosine triphosphate (ATP), and fibrinogen were normal. Blood samples for genetic studies were available from his parents, who are first cousins, and 9 relatives (2 grandparents and 7 brothers and sisters), with negative, mild, or severe bleeding history. The 57-year-old brother, with severe bleeding history (III-1, Figure 1), also provided blood samples for platelet function studies.
A total of 36 healthy subjects, with no personal history of abnormal bleeding, were also studied. All subjects gave consent to the study.
Materials
[3H]PSB-0413 was prepared by catalytic hydrogenation with tritium gas (GE Healthcare, Buckinghamshire, UK) of the propargyl precursor PSB-0412 as described.15 ADP, 2MeSADP, collagen, the thromboxane/prostaglandin endoperoxide analog 9,11-dideoxy-11,9-epoxymethano-prostaglandin F2, thrombin receptor activating peptide 6, PGE1, and PGI2, were from Sigma (St. Louis, MO). Apyrase was purified from potatoes.17 Commercial preparations of luciferin/luciferase reagent were used to measure the platelet ATP and ADP contents (ATP Assay Kit; BioOrbit Oy, Turku, Finland), and platelet secretion concurrently with platelet aggregation (Chronolume; Chrono-log Corp., Havertown, PA). Serum thromboxane B2 was measured by a commercially available enzymatic immunoassay (Thromboxane B2 EIA kit; Cayman Chemical Co., Ann Arbor, MI).
Preparation of platelet-rich plasma (PRP) and washed platelet suspensions
For studies with PRP, 9 volumes of blood were drawn into 1 volume of 109 mmol/L trisodium citrate and then centrifuged at 200g for 10 minutes.18,19 The supernatant PRP was transferred into a clean plastic tube; the platelet counts in PRP samples were adjusted to 2.5 × 1011/L in experiments performed in Freiburg (Germany) and not adjusted to a predefined value in experiments performed in Milan (Italy) and Strasbourg (France).18,20 For the preparation of washed platelet suspensions, 6 volumes of blood were drawn into 1 volume of acid-citrate-dextrose anticoagulant, centrifuged at 200g for 10 minutes to obtain PRP, which was used to prepare twice-washed platelet suspensions, according to the method described by Mustard et al,21 with the exception that 500 nmol/L PGI2 was added during the first and second washes. Platelet counts in washed platelet suspensions were adjusted to 3 × 1011/L.
Studies of platelet aggregation and secretion
The first screening of platelet aggregation in patient III-7 was performed in Freiburg (Germany), using the light transmission aggregometer APACT 4 (Labor Fibrintimer, Ahrensburg, Germany), and collagen (Nycomed, Austria) and ADP (MP Biomedicals) as agonists. Two separate sets of experiments were subsequently done in Milan (Italy), where platelet aggregation and secretion were studied simultaneously by lumi-aggregometry. Samples of PRP (0.45 mL) were incubated with 50 μL luciferine/luciferase reagent at 37°C for 30 seconds and stirred at 1000 rpm in a lumi-aggregometer (Lumi-aggregometer; Chrono-log Corp.). After incubation, 10 μL of an aggregating agent was added, and the aggregation and ATP secretion tracings were recorded for 3 minutes. Platelet aggregation of patient III-1 was studied in Strasbourg (France) in an APACT 4004 aggregometer.
Binding of fluorescein-conjugated fibrinogen to activated platelets
To measure the binding of fibrinogen to activated platelets, aliquots of 100 µL PRP (5 × 1010/L) were mixed with fluorescein-conjugated fibrinogen (final concentration 150 µg/mL) and were stimulated with different concentrations of ADP (0.25-2.0 µmol/L). After 3 minutes incubation, 100 µL 1% formaldehyde was added at room temperature. Platelets were then washed with phosphate-buffered saline and then diluted in 500 µL phosphate-buffered saline for analysis on FACS Calibur (Becton Dickinson, Germany). Histograms were obtained from 5000 cells, and the data were analyzed using the software CellQuest Pro (Becton Dickinson). Mean fluorescence intensities (MFIs) vs cell number were expressed in a linear mode.
Binding of [3H]PSB-0413 to washed platelets
Binding experiments were performed using [3H]PSB-0413, which is a tritiated derivative of a selective nucleotide antagonist of the P2Y12R, AR-C67085MX [2-propylthioadenosine-5′-adenylic acid (1,1-chloro-1-phosphonomethyl-1-phosphonylanhydride)], and 9 × 107 washed platelets.15 Nonspecific binding was defined in the presence of 1 mM ADP. Washed platelets were incubated with the ligand at 37°C for 5 minutes; then bound and free radioactivity was separated by filtration through Whatman GF/B glass-fiber filters. Filters were then washed with 5 × 2 mL of ice-cold washing buffer (Tris HCl, 50 mM, pH 7.5; EDTA, 1 mM; MgCl2, 5 mM; NaCl, 100 mM). Filter-bound radioactivity was counted in 2-mL liquid scintillation counter. Assays were performed in triplicate in 3 independent experiments, 2 in III-7 and 1 in III-1.
Competition experiments were performed in triplicate using a fixed concentration of [3H]PSB-0413 (200 nM) and increasing concentrations of ADP (10−10-10−2 M) or 2MeSADP (10−11-10−4 M) at 37°C for 5 minutes.
Measurement of platelet cAMP
Platelet cAMP was measured by a radioisotopic assay, using a commercially available kit (Cyclic AMP [3H] assay system; Amersham International, UK). Duplicate samples of 1 mL citrated PRP containing 1 mM theophylline were incubated with Tyrode’s buffer and PGE1 (1 μM), PGE1 and ADP or epinephrine (0.1 and 1.0 μM), or Tyrode’s buffer alone in a control mixture. After incubation at 37°C (2 minutes), 1 mL of 5% trichloroacetic acid was added, and the samples were snap-frozen in dry ice and methanol, thawed at ambient temperature, and then shaken at 4°C for 45 minutes. After centrifugation at 4°C for 30 minutes, the supernatant was extracted 3 times with 5 mL of water-saturated ether, dried under a stream of nitrogen at 60°C, and stored at −20°C. Before assay, the samples were reconstituted with 0.05 mol/L Tris buffer containing 4 mmol/L EDTA.
VASP phosphorylation analysis
The VASP phosphorylation analysis was performed immediately after blood collection, using Platelet VASP (Diagnostica Stago, Asnières, France) according to the manufacturer’s instructions. Briefly, blood samples were incubated with PGE1 and ADP, alone and in combination, for 10 minutes and fixed with paraformaldehyde. Platelets were then permeabilized with a nonionic detergent, labeled with a primary monoclonal antibody (16C2), followed by a secondary fluorescein isothiocyanate-conjugated polyclonal goat anti-mouse Ig antibody. Analyses were performed using a Becton Dickinson (Plymouth, UK) FACS Calibur flow cytometer. The platelet population was identified from its forward and side scatter distribution, and 10 000 platelets were gated. Platelet reactivity index (PRI) was calculated from the MFI, reflecting VASP phosphorylation, of samples incubated with PGE1 or PGE1 plus ADP, according to the following formula: PRI = [(MFI(PGE1) – MFI(PGE1 + ADP))/MFI(PGE1)] × 100. The analysis was completed in ∼30 minutes following blood sampling.
Genetic studies
Genomic DNA was isolated from peripheral blood lymphocytes using standard procedures. The entire coding sequence of the P2Y12R gene was amplified by a single polymerase chain reaction (PCR). PCR was performed using the primers (forward) 5′CCTTAGGCTGAAAATAACCATCCTC3′ and (reverse) 5′GCGCTTTGCTTTAACGAGTTCTGAAC3′, with DreamTaq Green Polymerase, 10× DreamTaq Green (Fermentas ThermoFisher Scientific Inc., Waltham, MA) and 2′-deoxynucleoside 5′-triphosphate mix (Invitrogen-Life Technologies Corp., Carlsbad, CA). The amplified fragments were subjected to DNA sequence analysis by ABI 3730xl 96-capillary DNA Analyzers (Eurofins MWG Operon, Huntsville, AL).
Molecular modeling of the P2Y12R
The recently resolved crystallographic structures of the human P2Y12R in complex with the antagonist AZD1283 (Protein Data Bank ID: 4NTJ)12 and with the full agonist 2MeSADP (Protein Data Bank ID: 4PXZ)13 were used in this study. Models of the P2Y12R Gln-187 variant, based on the agonist-bound P2Y12R structure, were built by mutating His187 to Gln directly in the structure and then by modifying its conformation by selecting 5 different, suitable rotamers.
All P2Y12R structures were prepared using the Protein Preparation Wizard tool22 implemented in the Schrödinger suite,23 adding all the hydrogen atoms and the missing side chains of residues whose backbone coordinates were observed in the structure. The orientation of polar hydrogens was optimized, the protein protonation states were adjusted, and the overall structures were minimized with harmonic restraints on the heavy atoms, to remove strain. Then, all the heterogroups and water molecules were deleted.
The SiteMap tool of the Schrödinger suite was used to identify potential binding sites in the structures. Molecular docking of PSB-0413 at the agonist-bound P2Y12R crystal and at the P2Y12 Gln-187 variant models was performed by means of the Glide package24 from the Schrödinger suite.23 In particular, a Glide Grid was centered on the centroid of residues located within 6 Å from the previously identified cavity. The Glide Grid was built using an inner box (ligand diameter midpoint box) of 14 Å × 14 Å × 14 Å and an outer box (box within which all the ligand atoms must be contained) that extended 20 Å in each direction from the inner one. Docking of the ligand was performed in the rigid binding sites using the XP (extra precision) procedure. The top-scoring docking poses of the ligand were subjected to visual inspection and analysis of protein-ligand interactions to select the final binding conformations.
Results
Studies in patient III-7 (propositus)
Platelet aggregation and secretion.
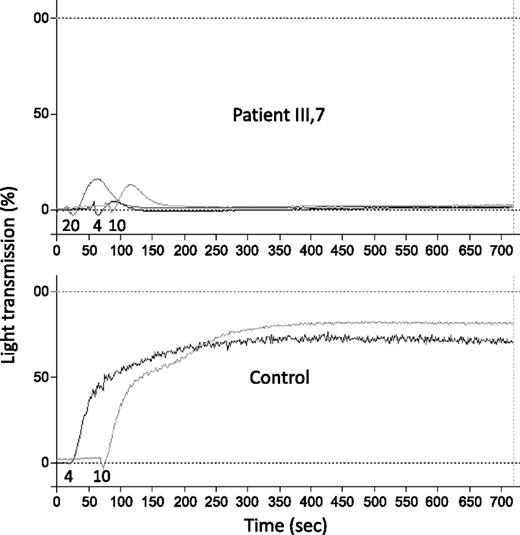
The platelets from patient III-7 changed shape normally following stimulation with 1 μM ADP (not shown) and underwent aggregation in response to 4-20 μM ADP that was markedly lower than normal and rapidly reversible (Figure 2). The aggregation of these platelets in response to other platelet agonists was normal (not shown). Very similar results were obtained in experiments performed in Freiburg (Figure 2) and in Milan (not shown). The ATP secretion from patient platelets was absent after stimulation by ADP, and normal after stimulation by other agonists (Table 1).
ADP-induced platelet aggregation in PRP from a normal control and from patient III-7. Numbers below the aggregation tracings indicate the concentration of ADP added (µM). These experiments were performed using the light transmission aggregometer APACT 4 (Labor Fibrintimer).
ADP-induced platelet aggregation in PRP from a normal control and from patient III-7. Numbers below the aggregation tracings indicate the concentration of ADP added (µM). These experiments were performed using the light transmission aggregometer APACT 4 (Labor Fibrintimer).
Secretion of platelet ATP induced by different agonists in patient III-7
| Agonist . | Released ATP (nmol/108 platelets) . | ||
|---|---|---|---|
| Patient III-7, experiment 1 . | Patient III-7, experiment 2 . | Normal range* . | |
| ADP (4 μM) | Undetectable | Undetectable | 0.02-0.98 (n = 96) |
| ADP (20 μM) | Undetectable | Undetectable | 0.04-0.61 (n = 59) |
| Collagen (2 μg/mL) | 0.46 | 0.47 | 0.17-0.93 (n = 62) |
| U46619 (0.5 μM) | 0.02 | 0.03 | 0.02-1.03 (n = 72) |
| TRAP-6 (10 μM) | Not done | 0.17 | 0.01-1.08 (n = 41) |
| Agonist . | Released ATP (nmol/108 platelets) . | ||
|---|---|---|---|
| Patient III-7, experiment 1 . | Patient III-7, experiment 2 . | Normal range* . | |
| ADP (4 μM) | Undetectable | Undetectable | 0.02-0.98 (n = 96) |
| ADP (20 μM) | Undetectable | Undetectable | 0.04-0.61 (n = 59) |
| Collagen (2 μg/mL) | 0.46 | 0.47 | 0.17-0.93 (n = 62) |
| U46619 (0.5 μM) | 0.02 | 0.03 | 0.02-1.03 (n = 72) |
| TRAP-6 (10 μM) | Not done | 0.17 | 0.01-1.08 (n = 41) |
PRP containing trisodium citrate as anticoagulant was stimulated by different agonists at the indicated concentrations.
TRAP-6, thrombin receptor activating peptide 6; U46619, thromboxane/prostaglandin endoperoxide analog 9,11-dideoxy-11,9-epoxymethano-prostaglandin F2.
The normal ranges are defined by the 2.5 and 97.5 percentiles of the distribution of values obtained in healthy subjects.
Binding of fluorescein-conjugated fibrinogen to ADP-activated platelets.
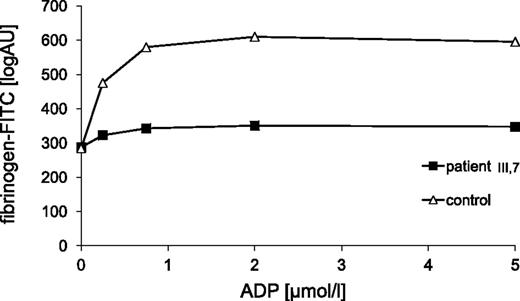
Compared with healthy controls, the amount of fluorescein-conjugated fibrinogen bound to platelets upon stimulation with ADP (0.25-2.0 µmol/L) was severely impaired (Figure 3).
Binding of fluorescein-conjugated fibrinogen to ADP-activated platelets from a healthy control and patient III-7. Fluorescein-conjugated (FITC) fibrinogen binding to PRP stimulated with the indicated concentrations of ADP was measured by flow cytometry (see “Methods” for details). FITC, fluorescein isothiocyanate.
Binding of fluorescein-conjugated fibrinogen to ADP-activated platelets from a healthy control and patient III-7. Fluorescein-conjugated (FITC) fibrinogen binding to PRP stimulated with the indicated concentrations of ADP was measured by flow cytometry (see “Methods” for details). FITC, fluorescein isothiocyanate.
Inhibition of PGE1-induced increase in platelet cAMP.
The basal level of platelet cAMP was normal (12.2 pmol/109 platelets; normal range: 6-17.9) and normally increased ∼10-fold after stimulation with 1 μM PGE1. ADP, in a concentration-dependent manner, inhibited the production of cAMP by normal platelets exposed to PGE1, but this response was greatly impaired in the patient’s platelets (Table 2). In contrast to ADP, epinephrine normally inhibited the production of cAMP in the patient’s platelets exposed to PGE1 (Table 2), suggesting that the defect could be attributed to an abnormality in the platelet P2Y12R responsible for specifically coupling ADP stimulation to adenylyl cyclase inhibition.
Effects of ADP and epinephrine on PGE1 (1 µM)–induced increase of cAMP levels in platelets from patient III-7 and healthy controls
| . | cAMP (% of PGE1-induced) . | ||
|---|---|---|---|
| Patient III-7, experiment 1 . | Patient III-7, experiment 2 . | Healthy controls* (n = 21) . | |
| ADP (0.1 μM) | 90.0 | 92.8 | 78.62 ± 11.58 |
| ADP (1.0 μM) | 107.0 | 100.0 | 44.41 ± 11.88 |
| Epinephrine (1 μM) | 22.0 | 26.0 | 30.36 ± 13.94 |
| . | cAMP (% of PGE1-induced) . | ||
|---|---|---|---|
| Patient III-7, experiment 1 . | Patient III-7, experiment 2 . | Healthy controls* (n = 21) . | |
| ADP (0.1 μM) | 90.0 | 92.8 | 78.62 ± 11.58 |
| ADP (1.0 μM) | 107.0 | 100.0 | 44.41 ± 11.88 |
| Epinephrine (1 μM) | 22.0 | 26.0 | 30.36 ± 13.94 |
PRP containing trisodium citrate as anticoagulant was exposed to PGE1 (1 μM) in the presence or absence of ADP or epinephrine at the indicated concentrations for 2 minutes at 37°C (see “Methods” for details).
Means ± SD.
VASP phosphorylation assay.
The PRI value of patient’s platelets was 30% in an experiment done in Milan and 1% in a subsequent experiment done in Freiburg, compared with 78.7 ± 6.7% in platelets from 31 normal subjects.
Binding of [3H]PSB-0413 to washed platelet suspensions.
The binding of [3H]PSB-0413 to patient platelets was measured in 2 separate experiments and compared with that observed in normal volunteers. The specific binding was saturable in both cases, and Scatchard plot analysis yielded a linear fit. The number of binding sites per platelet (Bmax) was comparable between normal and patient platelets (Table 3); however, the dissociation constant (KD) values were higher with patient platelets than with normal platelets (Table 3).
Binding studies of [3H]PSB-0413 on intact platelets from the propositus (III-7) and his brother (III-1) and 10 healthy controls
| . | Patient III-7, experiment 1 . | Patient III-7, experiment 2 . | Patient III-1 . | Healthy controls* (n = 10) . |
|---|---|---|---|---|
| Bmax (sites per platelet) | 285 | 436 | 693 | 425 ± 50 |
| KD (nM) | 8.4 | 33.0 | 19.0 | 3.3 ± 0.6 |
| . | Patient III-7, experiment 1 . | Patient III-7, experiment 2 . | Patient III-1 . | Healthy controls* (n = 10) . |
|---|---|---|---|---|
| Bmax (sites per platelet) | 285 | 436 | 693 | 425 ± 50 |
| KD (nM) | 8.4 | 33.0 | 19.0 | 3.3 ± 0.6 |
Means ± SD.
Characterization of the P2Y12R gene.
The observation that the patient platelets had a normal number of binding sites for [3H]PSB-0413 in spite of a severely impaired function of the P2Y12R suggested that a dysfunctional receptor was being synthesized in normal amounts. To identify the underlying structural changes, we analyzed the coding sequence of P2Y12R from DNA fragments generated in a PCR reaction. DNA from the patient showed a homozygous c.847T>A substitution that changed the codon for His-187 to Gln (p.His187Gln), located in the TM5 portion of the receptor.
Family studies
For 11 of 20 first- and second-degree relatives of the propositus, it was possible to retrieve information on the individual bleeding history and blood samples for DNA extraction and genotyping. Seven subjects (the grandparents, the parents, 2 sisters, and 1 brother) were heterozygous for p.His187Gln, 1 brother and 2 sisters had wild-type (WT) P2Y12R, and 1 brother had homozygous p.His187Gln. The bleeding history was negative in all subjects, except in 2 sisters (1 with heterozygous p.His187Gln P2Y12R, 1 with WT genotype) who had a mild bleeding history, and in the brother (III-1) with homozygous p.His187Gln P2Y12R, who had a severe bleeding history.
Patient III-1 underwent extensive platelet function studies in Strasbourg (France), including platelet aggregation both in PRP and washed platelet suspensions, inhibition of PGE1-induced increase in platelet cAMP levels by ADP, and VASP phosphorylation assay. The results of these tests were superimposable to those obtained in the propositus and compatible with severe defect of P2Y12R function. The VASP-P PRI was 1% in 2 separate experiments, similarly to that obtained in patient III-7 in Freiburg; ADP did not inhibit PGE1-induced increase in cAMP up to a concentration of 100 μM. Similarly to the propositus, binding studies of [3H]PSB-0413 to his platelets revealed that the Bmax was normal (693 sites per platelet), whereas the KD value was higher than normal (19.0 nM) and comparable to the mean KD value calculated in the 2 experiments with the proband’s platelets. The binding affinity (Ki) values for both ADP and 2MeSADP for bound radioligand were higher in patient III-1 than in a healthy control (Figure 4).
Competition of ADP and 2MeSADP for binding of [3H]PSB-0413 to washed platelets from patient III-1 and a healthy control. Higher concentrations of either ADP or 2MeSADP were necessary to displace [3H]PSB-0413 from the patient’s platelets, compared with control.
Competition of ADP and 2MeSADP for binding of [3H]PSB-0413 to washed platelets from patient III-1 and a healthy control. Higher concentrations of either ADP or 2MeSADP were necessary to displace [3H]PSB-0413 from the patient’s platelets, compared with control.
Finally, the results of preliminary experiments using 1321N1 astrocytoma cells expressing p.His187Gln P2Y12R were similar to those obtained in patients’ platelets: compared with 1321N1 astrocytoma cells expressing WT P2Y12R, we observed decreased affinity for [3H]PSB-0413 and a right shift of the displacement curves using ADP and 2MeSADP (data not shown).
Molecular modeling studies
To predict the implications of the p.His187Gln substitution at a molecular level, we performed molecular modeling studies of the P2Y12R. Two recently published crystallographic structures of the human P2Y12R,12,13 in complex with both agonist and antagonist, resulted to be very useful for this study. Moreover, models of the P2Y12R p.His187Gln variant were built using the agonist-bound P2Y12R crystal structure, to analyze the possible structural differences because of this sequence variation.
TM5 in both P2Y12R crystal structures is unusually straight and tilted, because of the absence of proline (eg, position 5.50 following the Ballesteros-Weinstein numbering system)25 or glycine residues in this helix in other G-protein-coupled receptors that destabilize its straight α-helical conformation. Previous modeling attempts of the P2Y12R based on the available crystallographic templates are imprecise because of this difference.
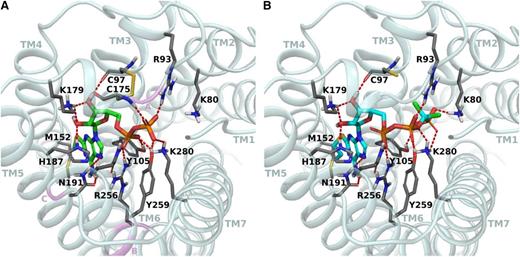
In both structures, the point of mutation is located in the upper part of TM5 facing inward toward the binding site, in proximity of the ligand. Comparison of the 2 different crystallographic structures, agonist and antagonist bound, revealed large conformational changes occurring in TM6 and TM7 but also a slightly different orientation of TM5. Consequently, the conformations of the residues in this helix, including His187 (5.36), differ in the 2 structures. In the WT antagonist-bound P2Y12R structure, the side chain of His187 is more oriented toward TM4 and is delimiting the binding site making hydrophobic interactions with the ligand AZD1283. In the WT agonist-bound P2Y12R structure, the side chain of His187 (5.36) is directly interacting through an H-bond with the 2′-hydroxyl group of 2MeSADP (Figure 5). Moreover, the aromatic ring of this histidine is perpendicular to the adenine core and can stabilize it through hydrophobic interactions. Docking of PSB-0413 at this WT P2Y12R structure closely resembles the binding mode of 2MeSADP (Figure 5). In fact, the adenine bases of the 2 ligands occupy a common aromatic binding site, forming a π-π interaction with Tyr105 (3.33) and H-bonds with Asn191 (5.40). The 2-propylthio group of PSB-0413 extends deeper in the same hydrophobic pocket occupied by the 2-methylthio group of the crystallized agonist and delimited by Phe106 (3.34), Met152 (4.53), Leu155 (4.56), Ser156 (4.57), Val190 (5.39), and Cys194 (5.43). The ribose 2′- and 3′-hydroxyl groups form H-bonding interactions with Lys179 (EL2) and His187 (5.36) and with Lys179 (EL2) and the backbone carbonyl of Cys97 (3.25), respectively. The noncleavable triphosphate-like chain of PSB-0413 is directed toward TM2 and forms numerous hydrophilic and ionic interactions with Lys80 (2.60), Arg93 (3.21), Arg256 (6.55), Tyr259 (6.58), and Lys280 (7.35).
Binding site of the human P2Y12R. Comparison of the crystallographic pose of 2MeSADP (green carbons) (A) and the docking pose of PSB-0413 (cyan carbons) (B) at the WT agonist-bound P2Y12R crystal structure. Side chains of some amino acids important for ligand recognition are highlighted (gray carbon sticks). H-bonding and ionic interactions are pictured as red dotted lines. Nonpolar hydrogen atoms are not displayed. In panel A, ribbon regions corresponding to residues whose mutation is associated with a receptor dysfunction previously reported in patients are highlighted in pink and labeled with pink letters as follows: A, Lys174; B, Arg256, Pro258; and C, Arg265.
Binding site of the human P2Y12R. Comparison of the crystallographic pose of 2MeSADP (green carbons) (A) and the docking pose of PSB-0413 (cyan carbons) (B) at the WT agonist-bound P2Y12R crystal structure. Side chains of some amino acids important for ligand recognition are highlighted (gray carbon sticks). H-bonding and ionic interactions are pictured as red dotted lines. Nonpolar hydrogen atoms are not displayed. In panel A, ribbon regions corresponding to residues whose mutation is associated with a receptor dysfunction previously reported in patients are highlighted in pink and labeled with pink letters as follows: A, Lys174; B, Arg256, Pro258; and C, Arg265.
When docked at the P2Y12R p.His187Gln variant models, PSB-0413 showed the same orientation in the binding site and similar interactions as in the WT receptor (data not shown). However, depending on the rotamer of the side chain of Gln187, the H-bonding network involving the 2′-hydroxyl group can differ. In fact, in some rotamers Gln187 does not form any H-bond with the ligand, while in others it can act as H-bond acceptor or H-bond donor.
Discussion
Congenital defects and pharmacologic inhibition of the P2Y12R for ADP are associated with an increased bleeding risk, demonstrating the important role of this receptor in platelet function and normal hemostasis.26 Most of the congenital defects of P2Y12R characterized to date are associated with a marked decrease of the platelet binding sites for ADP, which has been attributed to mutations that disrupt the synthesis of the receptor,2,26-30 to alterations in the ligand binding site,10 or to compromised P2Y12R recycling.14 A recently described patient displayed abnormalities in P2Y12R signaling, traffic, and surface expression, which was associated with the substitution of Arg122 with a cysteine.31 In 1 kindred, a case of a normal expression of the receptor that was associated with severely defective signal transduction was described.7 Nucleotide sequence analysis of the P2Y12 gene revealed that the patient was a compound heterozygote for 2 missense mutations that caused the substitution p.Arg256Gln (in TM6) and p.Arg265Trp (in EL3), suggesting that the structural integrity of TM6 and EL3 regions is necessary for normal receptor function. A heterozygous mutation in the same region of the molecule, p.Pro258Thr, was later described in a patient with mild bleeding disorder and severely impaired ADP-induced platelet aggregation, confirming that this region of the molecule is important for receptor function.8 The recently reported crystallographic data for the human P2Y12R support these findings revealing that significant conformational changes occur in the TM6-EL3 region between the agonist- and antagonist-bound structures.12,13
In the present report, we describe the case of a patient and his brother with lifelong bleeding disorder associated with defective P2Y12R-dependent platelet function, associated with normal expression of the receptor but decreased affinity for its ligand. Based on the results of the VASP phosphorylation assay6 and on the observation that ADP, up to 100 μM, failed to inhibit adenylyl cyclase, we may conclude that the function of the mutant receptor is completely suppressed. The moderately reduced PRI value (30%) initially measured by the VASP-P assay in patient III-7 was not reproduced in a second experiment (PRI = 1%) and in 2 experiments performed in patient III-1, suggesting that the results of the first experiment were likely inaccurate.
The bleeding histories of our proband and his brother were clinically relevant and comparable to that of patients with severe P2Y12R deficiency, associated with homozygous base pair deletions in the open reading frame, resulting in frame shifts and premature truncation of the protein.5 The study of many relatives of the propositus, whose parents were first cousins, depicts a typical inheritance pattern of recessive autosomal diseases. The bleeding history of relatives with heterozygous p.His187Gln P2Y12R was essentially absent, comparable to that of subjects with the WT receptor, confirming previous observations.2
In order to analyze the structural implications of the p.His187Gln mutation, molecular modeling studies were performed. Recently published crystallographic structures of the P2Y12R12,13 revealed that His187 in TM5 is oriented toward the inside of the receptor within the ligand binding site. In particular, the 2MeSADP-bound structure showed a clear role of His187 (5.36) in agonist binding because of the formation of a H-bond with the ligand’s 2′-hydroxyl group and hydrophobic interactions with adenine. PSB-0413 docking predicted a binding mode similar to the crystallographic pose of the nucleotide agonist. Therefore, the predicted involvement of His187 (5.36) also in PSB-0413 binding can explain the decreased affinity of [3H]PSB-0413 for the patient’s platelets, considering that the H187Q mutation can induce suboptimal H-bonding interactions with the ligand and attenuated stabilizing hydrophobic interactions. Moreover, the conformational changes highlighted by the comparison of the agonist- and antagonist-bound P2Y12R structures, involving mainly TM6 and TM7 and partially TM5, suggest that this region of the receptor is important for the activation process. Also, mutagenesis studies have shown the importance of residues located in the TM6-EL3 region for the functional integrity of the P2Y12R. Therefore, the p.His187Gln substitution could alter the interresidue interactions in this region as compared with WT, potentially interfering with conformational rearrangements occurring during the activation process.
In conclusion, His187 (5.36) is important for agonist and nucleotide antagonist (ie, PSB-0413) binding, as highlighted by crystallographic data and docking results, and located in a region undergoing conformational changes. Therefore, we have determined a structural context for the functional consequences of the p.His187Gln substitution in the P2Y12R.
There is an Inside Blood Commentary on this article in this issue.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgment
This work was supported by the National Institutes of Health National Institute of Diabetes and Digestive Kidney Diseases Intramural Research Program (K.A.J. and S.P.).
Authorship
Contribution: A.L. performed platelet function studies, analysis of the data, and final approval of the manuscript; C.R. performed DNA analysis, analysis of the data, and final approval of the manuscript; S.P. performed molecular modeling, analysis of the data, and final approval of the manuscript; A.D. and L.N. performed platelet function studies, analysis of the data, and final approval of the manuscript; P.O. performed ligand binding studies, analysis of the data, and final approval of the manuscript; C.G. performed platelet function studies, ligand binding studies, analysis of the data, and final approval of the manuscript; K.A.J. performed molecular modeling, analysis of the data, drafting of part of the manuscript, and final approval of the manuscript; B.Z. performed clinical management of the patients, platelet function studies, analysis of the data, and final approval of the manuscript; and M.C. performed coordination and design of the study, analysis of the data, drafting of the manuscript and final approval of the manuscript.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Marco Cattaneo, Unità di Medicina 3, Ospedale San Paolo – Università degli Studi di Milano, Via di Rudinì 8 20142 Milano, Italy; e-mail: marco.cattaneo@unimi.it.
References
Author notes
B.Z. and M.C. contributed equally to this study.

![Figure 4. Competition of ADP and 2MeSADP for binding of [3H]PSB-0413 to washed platelets from patient III-1 and a healthy control. Higher concentrations of either ADP or 2MeSADP were necessary to displace [3H]PSB-0413 from the patient’s platelets, compared with control.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/125/6/10.1182_blood-2013-07-517896/4/m_1006f4.jpeg?Expires=1769096879&Signature=VD7wOtxjsaypucYeKc~sjeKS5fvA4yrNJVTvqd6s7QubBVFmvkHRjP~8WiqWY3IS2f01UeyvVZy0f0Mb5FlbDaueCTrneirOct-8ohNAyUe66vp6wQs15TRFG37GCQyCY8WU-39FIUZT26zjPKIq6VIHjWXMdU91VRi5Bx9Fl5ZwDIhQA2aLusKQUSgwbgvx16QDf3IqoBnggT9DVWTiSN9~5CIwvGFa5MKMZmtGqA65jMicx1EKrQfIS3UK4mSMI2AuiB2fYMRKdyme0LyYLV4asvyh3usgAIOsO8riVxlPPxi99bN10Ms1fhr8YNrGvjlyV-gT8aEXa1YOZdOV0A__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
This feature is available to Subscribers Only
Sign In or Create an Account Close Modal